Proteopedia:Featured SEL/0
From Proteopedia
(Difference between revisions)
(New page: <table> <tr><td>Image:Oct2017.png</td></tr> <tr><td>'''HIV-1 protease'''</td></tr> <tr><td>''David Canner''</td></tr> <tr><td>The X-ray structure of HIV-1 protease reveals that it is c...) |
(image must be a link) |
||
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<table> | <table> | ||
| - | <tr><td> | + | <tr><td> |
| - | <tr><td>'''HIV-1 protease'''< | + | <imagemap> |
| - | + | Image:Anim HIV protease.gif|center | |
| - | + | default [[Immunodeficiency virus protease]] | |
| + | </imagemap> | ||
| + | </td></tr> | ||
| + | <tr><td><div class='scrolling '>'''HIV-1 protease'''<br> | ||
| + | ''by David Canner''<br> | ||
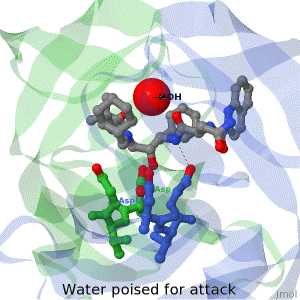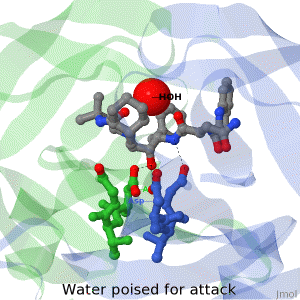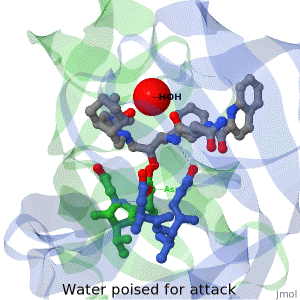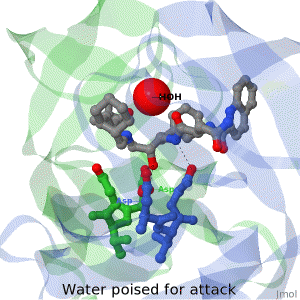
| + | The X-ray structure of HIV-1 protease reveals that it is composed of two symmetrically related subunits which form a tunnel where they meet. This is critical because it contains the active site of the protease, consisting on two Asp-Thr-Gly conserved sequences, making it a member of the aspartyl protease family. The two catalytic Asp's either interact with the incoming water or protonate the carbonyl to make the carbon more electrophilic for the incoming water. | ||
| + | |||
| + | >>> [[Immunodeficiency virus protease|Visit this page]] >>> | ||
| + | </div> | ||
| + | </td></tr> | ||
</table> | </table> | ||
| + | |||
| + | [[Category:Featured in Selected Pages]] | ||
Current revision
HIV-1 protease
by David Canner >>> Visit this page >>> |