Main Page
From Proteopedia
| Line 4: | Line 4: | ||
<span style="border:none; margin:0; padding:0.3em; color:#000; font-style: italic; font-size: 1.2em;"> | <span style="border:none; margin:0; padding:0.3em; color:#000; font-style: italic; font-size: 1.2em;"> | ||
| - | <b>Because life has more than 2D</b>, Proteopedia | + | <b>Because life has more than 2D</b>, Because life is more than 2D, Proteopedia aids in understanding the 3D relationships between function & structure of biomacromolecules |
| + | </span> | ||
Revision as of 07:46, 21 October 2018
|
ISSN 2310-6301
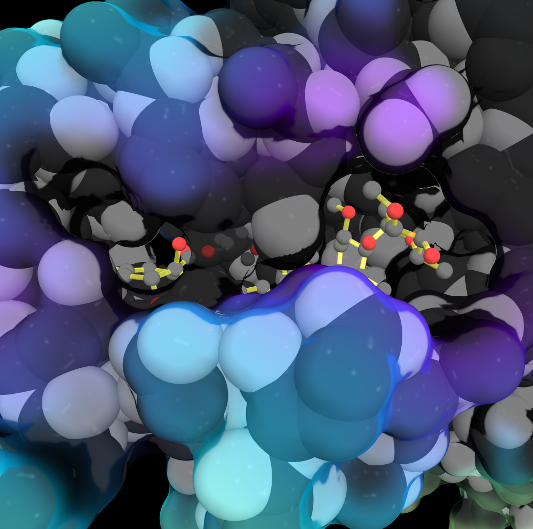
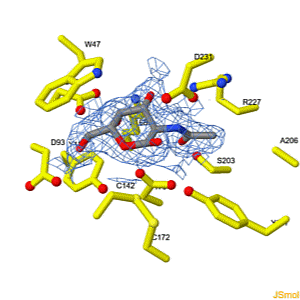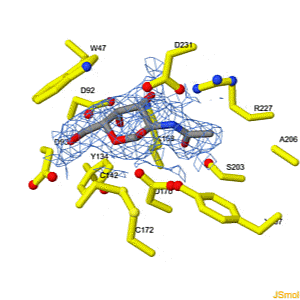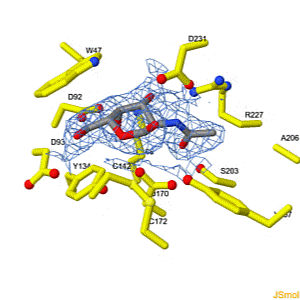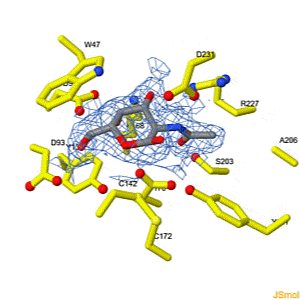
Because life has more than 2D, Because life is more than 2D, Proteopedia aids in understanding the 3D relationships between function & structure of biomacromolecules
| |||||||||||
| Selected Pages | Art on Science | Journals | Education | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
|
How to add content to Proteopedia Who knows ... |
Teaching Strategies Using Proteopedia |
||||||||||
| |||||||||||