User:Gisele A. Andree/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 42: | Line 42: | ||
'''Domain 5''' | '''Domain 5''' | ||
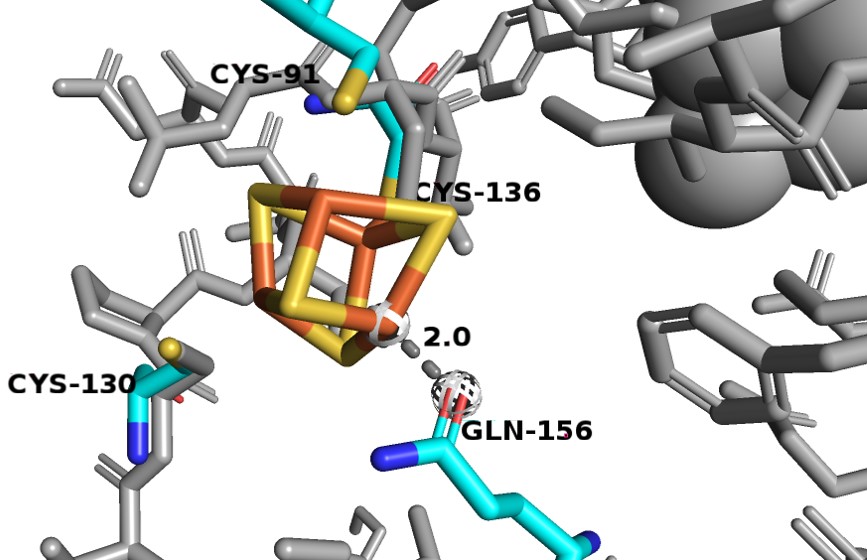
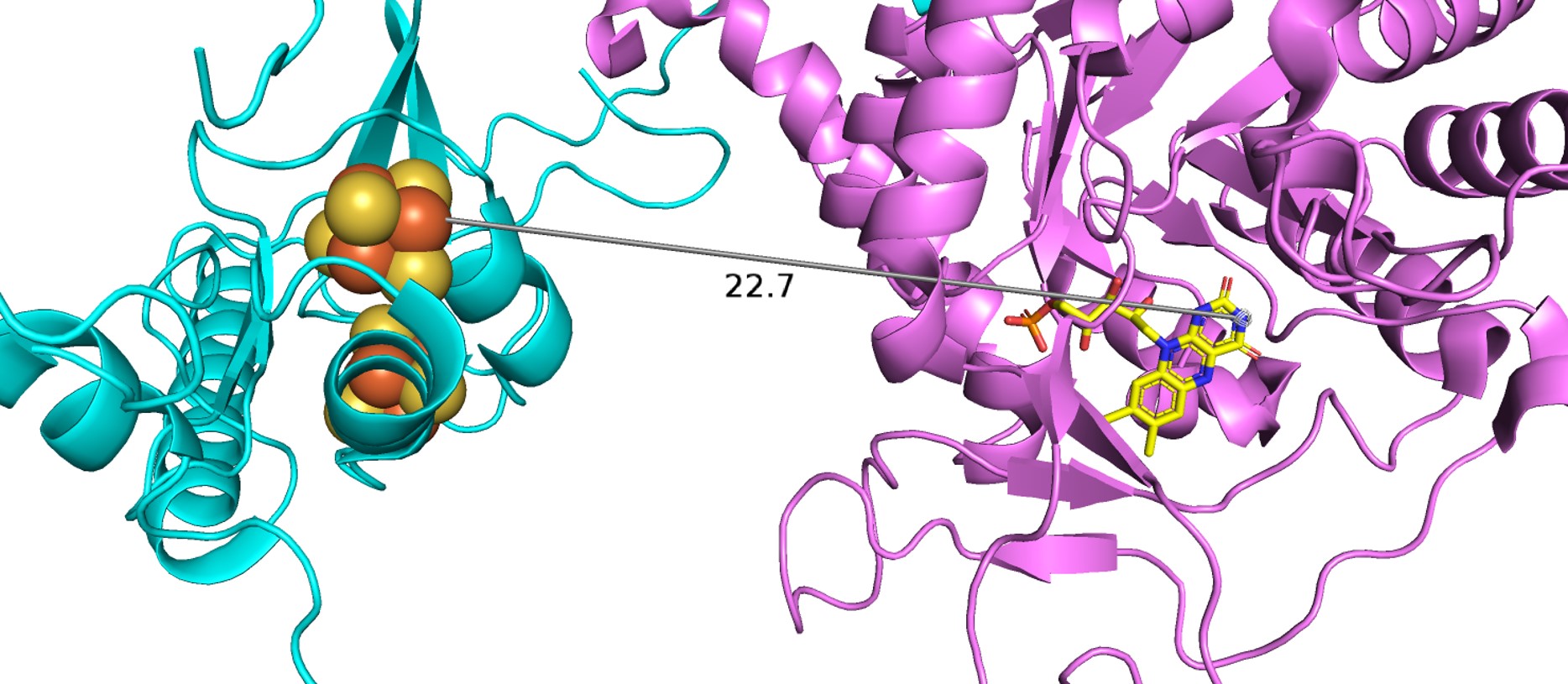
| - | Domain 5 consists of residues 1-26 and 848-1020. It also binds two [4Fe-4S] clusters. Between these clusters there are two α-helices and a four-stranded antiparallel β-sheet. The [4Fe-4S] clusters here in domian 5, which act as electron transfer centers, are not close to the next active redox site (the FMN binding site in domain 4). There is a gap of ~ | + | Domain 5 consists of residues 1-26 and 848-1020. It also binds two [4Fe-4S] clusters. Between these clusters there are two α-helices and a four-stranded antiparallel β-sheet. The [4Fe-4S] clusters here in domian 5, which act as electron transfer centers, are not close to the next active redox site (the FMN binding site in domain 4). There is a gap of ~23 Å between N-terminal domain 5 Fe-S clusters and the FMN in the domian 4 binding site, which is too long for electrons to be able to cross. However, in dimer confirmation, the redox partners are close enough for electrons to transfer, thus, the enzyme must be in dimer confirmation to be active. |
| + | |||
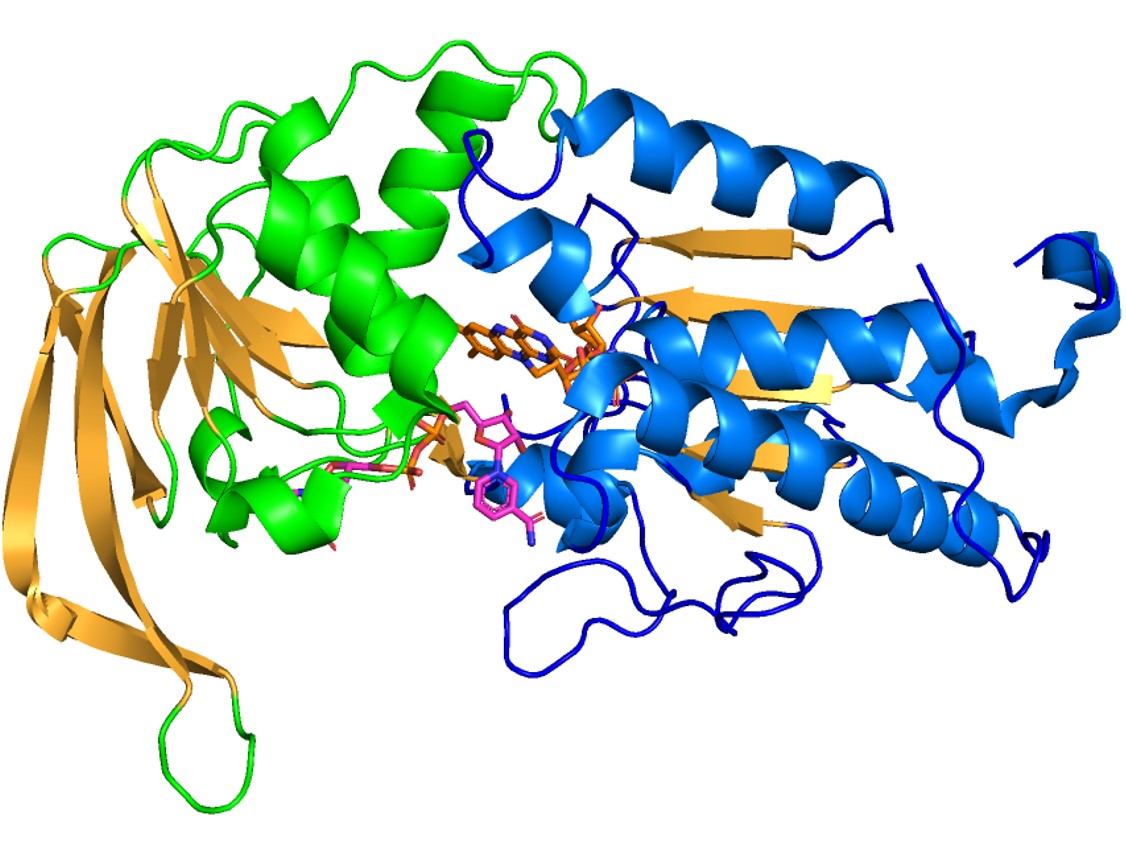
| + | Domain 5 is shown in cyan and domain 4 is shown in pink. The distance between the [4Fe-4S] clusters in domain 5 and the bound FMN (yellow) in domain 4 is indicated. | ||
[[Image:D5.jpg]] | [[Image:D5.jpg]] | ||
| Line 50: | Line 52: | ||
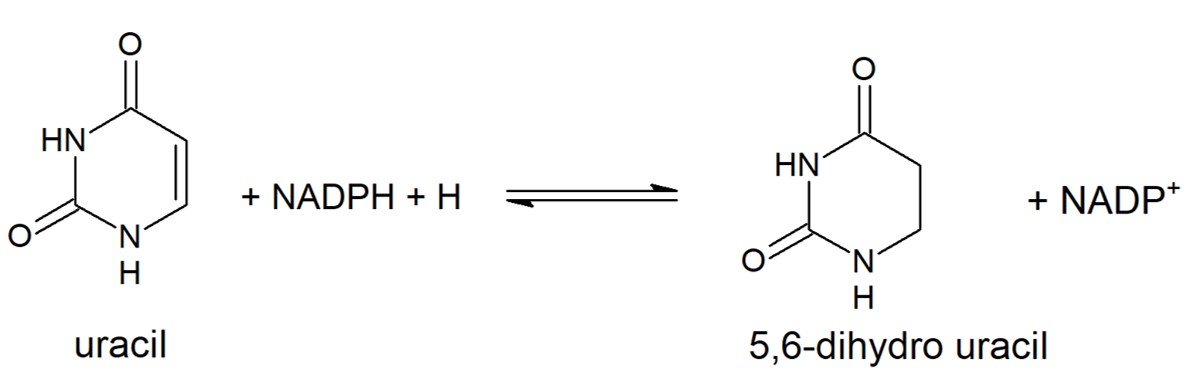
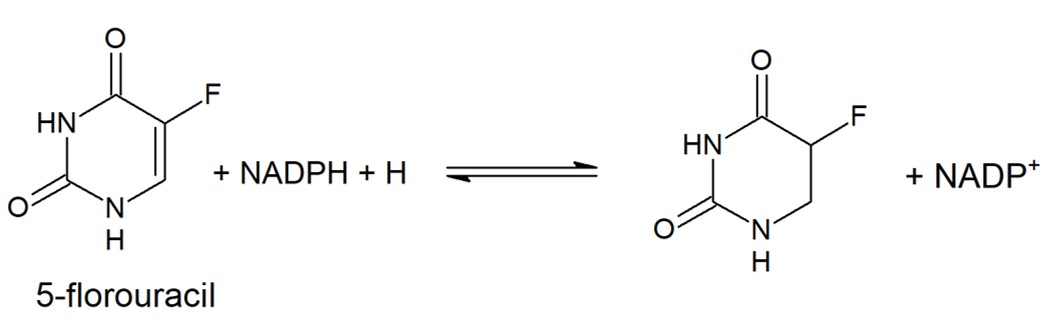
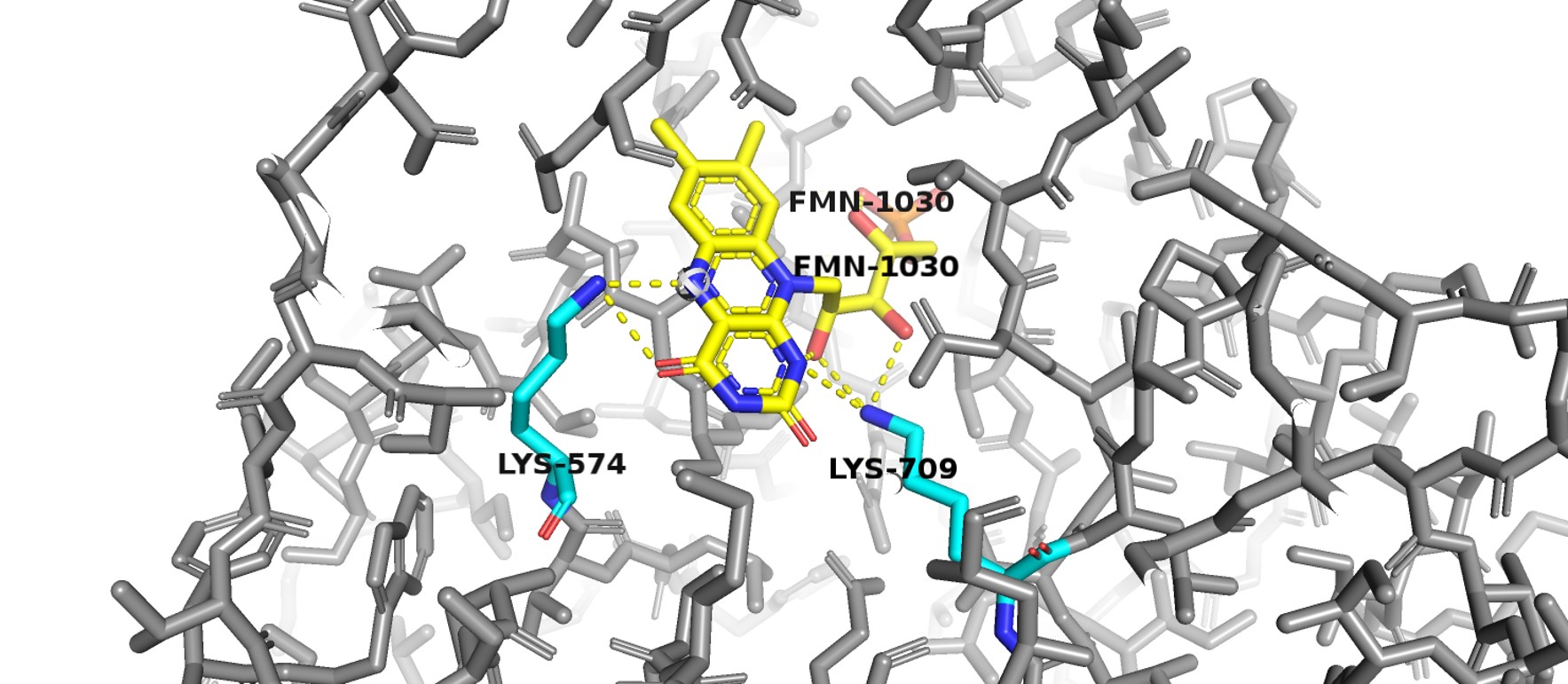
The enzyme has a two-site ping-pong mechanism with NADPH reducing FAD and reduced FMN responsible for reducing the pyrimidine. Transfer of electrons from NADPH to the pyrimidine is downhill with FMN rapidly reduced by FADH2 via the Fe-S conduit. Two single electrons are transferred via the Fe-S pathways. The reduction of the pyrimidine at the second active proceeds using general acid catalysis with protonation at N5 of FMN carried out by lysine-574, as FMN is reduced, and protonation at C5 of the pyrimidine by Cysteine-671, as it is reduced. | The enzyme has a two-site ping-pong mechanism with NADPH reducing FAD and reduced FMN responsible for reducing the pyrimidine. Transfer of electrons from NADPH to the pyrimidine is downhill with FMN rapidly reduced by FADH2 via the Fe-S conduit. Two single electrons are transferred via the Fe-S pathways. The reduction of the pyrimidine at the second active proceeds using general acid catalysis with protonation at N5 of FMN carried out by lysine-574, as FMN is reduced, and protonation at C5 of the pyrimidine by Cysteine-671, as it is reduced. | ||
| - | As earlier state, the dimer configuration is vitally important to the enzyme's function as electrons cannot travel through the Fe-S pathway without crossing over the dimer interface. In the isolated monomers, the gap between the N-terminal Fe-S clusters and FMN about | + | As earlier state, the dimer configuration is vitally important to the enzyme's function as electrons cannot travel through the Fe-S pathway without crossing over the dimer interface. In the isolated monomers, the gap between the N-terminal Fe-S clusters and FMN about 23 Å apart, too far for electrons to cross. As a result, all 12 redox co-factors of the DPD dimer are organized into two electron transfer chains passing the dimer interface twice. In this structural configuration, all redox partners are separated by ~7.5-10 Å, distances that are commonly observed in other multicenter electron transfer chains. Thus, DPD can only be active if it is in a dimer configuration and all of the Fe-S clusters participate in the electron transfer. |
[[Image:etransport]] | [[Image:etransport]] | ||
Revision as of 20:53, 13 December 2018
Structure of Eukaryotic Dihydropyrimidine Dehydrogenase
| |||||||||||
References
<Dobritzsch, D., Schneider, G., Schnackerz, K. D., & Lindqvist, Y. (2001). Crystal structure of dihydropyrimidine dehydrogenase, a major determinant of the pharmacokinetics of the anti‐cancer drug 5‐fluorouracil. The EMBO journal, 20(4), 650-660./> <Schnackerz, K. D., Dobritzsch, D., Lindqvist, Y., & Cook, P. F. (2004). Dihydropyrimidine dehydrogenase: a flavoprotein with four iron–sulfur clusters. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 1701(1-2), 61-74./>