Sandbox Reserved 1502
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
<StructureSection load='3lpt' size='340' side='right' caption='[[3lpt]], [[Resolution|resolution]] 2.00Å' scene=''> | <StructureSection load='3lpt' size='340' side='right' caption='[[3lpt]], [[Resolution|resolution]] 2.00Å' scene=''> | ||
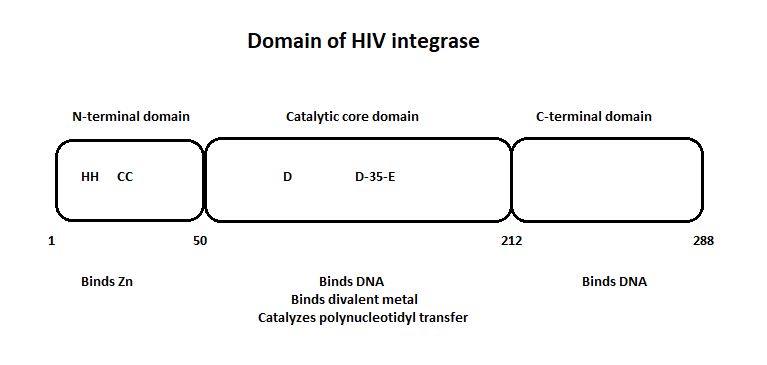
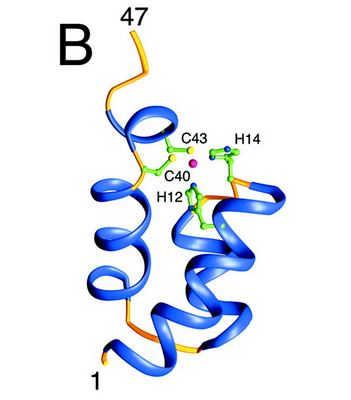
| - | The | + | The 3lpt is an integrase of the HIV, the Human Immunodeficiency Virus. An integrase is an enzyme produced by a retrovirus to integrate its genetic material into the DNA of the infected cell. It is one of three enzymes of HIV, the others being the Reverse Transcriptase and the Protease. |
== Structural highlights == | == Structural highlights == | ||
| Line 21: | Line 21: | ||
An integrase is an enzyme required for the integration of viral DNA into the host genome. The process of integration can be divided into two sequential reactions. In the first one, named 3'-processing, the enzyme removes di- or trinucleotides from viral DNA ends to expose 3′-hydroxyls attached to CA dinucleotides. The second step is the insertion of the processed 3′-viral DNA ends into the host chromosomal DNA by a trans-esterification reaction. This is called the strand transfer. | An integrase is an enzyme required for the integration of viral DNA into the host genome. The process of integration can be divided into two sequential reactions. In the first one, named 3'-processing, the enzyme removes di- or trinucleotides from viral DNA ends to expose 3′-hydroxyls attached to CA dinucleotides. The second step is the insertion of the processed 3′-viral DNA ends into the host chromosomal DNA by a trans-esterification reaction. This is called the strand transfer. | ||
| - | The HIV integrase is the enzyme responsible for the integration of the HIV's viral DNA into the host cell. | + | The HIV integrase is the enzyme responsible for the integration of the HIV's viral DNA into the host cell. |
| Line 44: | Line 44: | ||
====Structure and role of the core domain==== | ====Structure and role of the core domain==== | ||
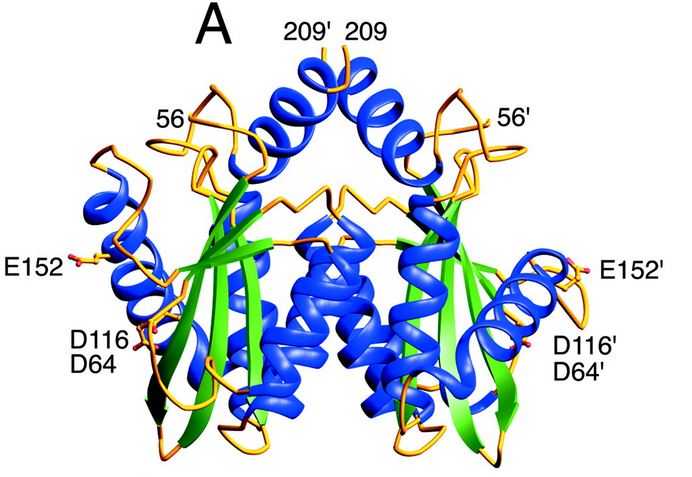
| - | The catalytic core domain contains three acidic residues, the D,D-35-E motif, comprising residues Asp64, Asp116, and Glu152. By analogy with models of catalysis by DNA polymerases, it has been proposed that coordination of divalent metal ion to these residues plays a key role in catalysis. The catalytic residues Asp64, Asp116, and | + | The catalytic core domain contains three acidic residues, the D,D-35-E motif, comprising residues Asp64, Asp116, and Glu152. By analogy with models of catalysis by DNA polymerases, it has been proposed that coordination of divalent metal ion to these residues plays a key role in catalysis. The catalytic residues Asp64, Asp116, and Glu152 of HIV integrase are in close proximity, coordinate divalent metal ion, and define the active site. The residues comprising the active site region exhibit considerable flexibility. That suggests that binding of DNA substrate is required to impose the precise configuration of residues that are required for catalysis. |
[[Image:Core enzyme.jpg]] [http://www.jbc.org/content/276/26/23213.full] | [[Image:Core enzyme.jpg]] [http://www.jbc.org/content/276/26/23213.full] | ||
Current revision
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
3lpt - HIV integrase
| |||||||||||