Substrate analogue complex structure of Mycobacterium tuberculosis decaprenyl diphosphate synthase
Tzu-Ping Ko, Xiansha Xiao, Rey-Ting Guo, Jian-Wen Huang, Weidong Liu, Chun-Chi Chen [1]
Molecular Tour
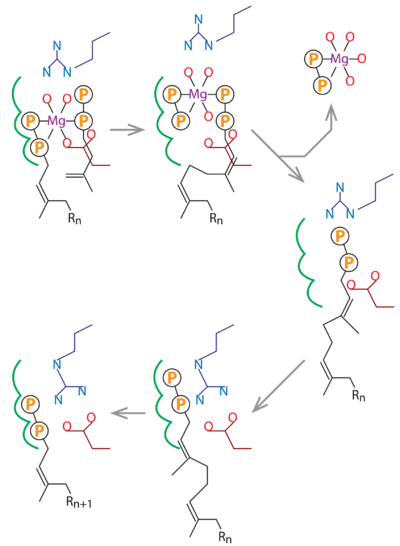
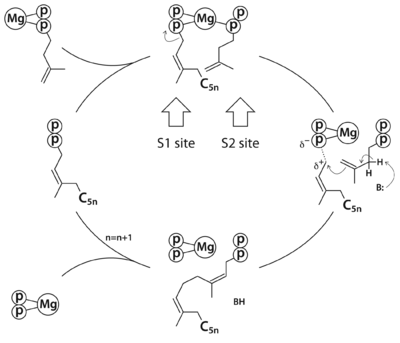
Rv1086 produces Ω-E,Z-farnesyl diphosphate (EZ-FPP, C15) from geranyl diphosphate (GPP, C10) and isopentenyl diphosphate (IPP, C5) that is used by Rv2361c for further elongation to form decaprenyl diphosphate (DPP, C50). In this structure we have substrate analogues of GPP and IPP as well as the essential Mg ion bound to Rv2361 (i.e. MtDPPS) in a productive mode. GPP binds to S1 site and IPP binds to S2 site. The hydrocarbon chains are joined head-to-tail to form a 5-carbon longer product. Meanwhile, we also have the GPP analogue bound in alternate conformations. The varying interactions of this substrate with Asp76 from one subunit and Arg292 from another may account for the transition pathway from S2 site to S1 site after each cycle of elongation reaction. So the enzyme can proceed to the next cycle of catalysis.
. The two monomers in an asymmetric unit of the MtDPPS crystal are shown as ribbon diagrams. The β-strands are named A-F and the α-helices numbered 1-7 from N to C terminus. They are colored yellow/red for one subunit and magenta/cyan for the other.
. The MtDPPS dimer is superimposed on itself with the two polypeptide chains switched. The protein is colored cyan/green in one dimer and pink/yellow in the other, and so are the side chains and the ligands, which are shown as stick models. Mg and water molecules are shown as spheres, and the coordinate bonds as dashed lines. Location of the S1 and S2 site as well as the nearby helices α1/α2 and strand βB are also indicated.
.
In this schematic diagram, the side chains of Asp76 and Arg292 are colored dark red and dark blue. The three subsites for the alternative binding modes of the S1 substrate are indicated by green curves. Other bonds, including the Mg-coordinates, are in black. Rn stands for a group of n consecutive isoprene units (C5n).
References
- ↑ Ko TP, Xiao X, Guo RT, Huang JW, Liu W, Chen CC. Substrate-analogue complex structure of Mycobacterium tuberculosis decaprenyl diphosphate synthase. Acta Crystallogr F Struct Biol Commun. 2019 Apr 1;75(Pt 4):212-216. PMID:30950820 doi:10.1107/S2053230X19001213