Dihydrolipoamide acetyltransferase
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
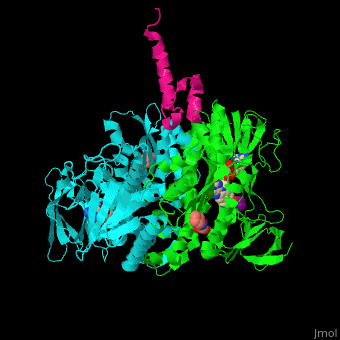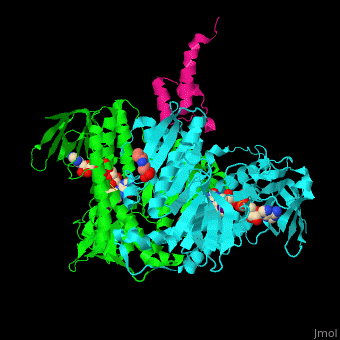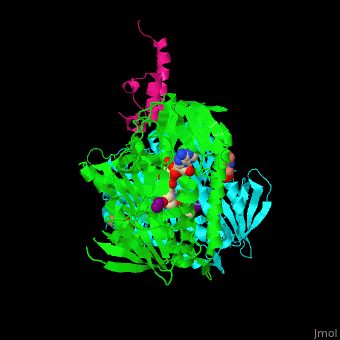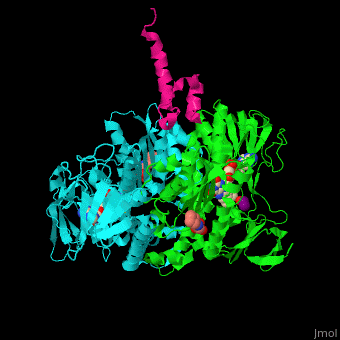
'''Dihydrolipoamide acetyltransferase''' (DLAT) or E2 is part of the pyruvate dehydrogenase complex together with pyruvate dehydrogenase (E1) and dihydrolipoyl dehydrogenase (E3). The complex decarboxylates pyruvate thus linking the glycolysis to the citric acid cycle. DLAT catalyzes the transfer of acetyl group to CoA.<ref>PMID:14736882</ref> | '''Dihydrolipoamide acetyltransferase''' (DLAT) or E2 is part of the pyruvate dehydrogenase complex together with pyruvate dehydrogenase (E1) and dihydrolipoyl dehydrogenase (E3). The complex decarboxylates pyruvate thus linking the glycolysis to the citric acid cycle. DLAT catalyzes the transfer of acetyl group to CoA.<ref>PMID:14736882</ref> | ||
| - | <scene name='48/485632/Cv/ | + | <scene name='48/485632/Cv/9'>Human dihydrolipoamide acetyltransferase binding domain with E3 binding domain, FAD, CHES and mercaptodthanol</scene>. |
| - | <scene name='48/485632/Cv/ | + | <scene name='48/485632/Cv/10'>FAD binding site</scene>. |
| - | <scene name='48/485632/Cv/ | + | <scene name='48/485632/Cv/11'>Mercaptodthanol binding site</scene>. |
| - | <scene name='48/485632/Cv/ | + | <scene name='48/485632/Cv/12'>CHES binding site</scene> (PDB entry [[3rnm]]), water molecules shown as red spheres. |
</StructureSection> | </StructureSection> | ||
Revision as of 10:33, 24 February 2019
| |||||||||||
3D structures of dihydrolipoamide acetyltransferase
Updated on 24-February-2019
References
- ↑ Lai WL, Chou LY, Ting CY, Kirby R, Tsai YC, Wang AH, Liaw SH. The functional role of the binuclear metal center in D-aminoacylase: one-metal activation and second-metal attenuation. J Biol Chem. 2004 Apr 2;279(14):13962-7. Epub 2004 Jan 21. PMID:14736882 doi:http://dx.doi.org/10.1074/jbc.M308849200