Aldolase
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
=Aldolase class II. Metal-dependent aldolase= | =Aldolase class II. Metal-dependent aldolase= | ||
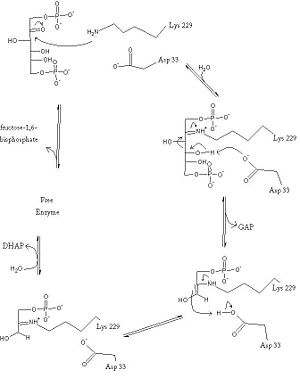
| - | ''' | + | '''Fructose-1,6-bisphosphate aldolase''' catalyzes the conversion of fructose-1,6-bisphosphatealdol to dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P) <ref>PMID:10712619</ref>.<br /> |
| + | '''Tagatose-1,6-bisphosphate aldolase''' catalyzes the aldol condensation of DHAP with G3P to produce tagatose 1,6-bisphosphate<ref>PMID:11940603</ref>.<br /> | ||
'''Fuculose-1-phosphate aldolase''' catalyzes the cleavage of fuculose-1-phosphate to dihydroxyacetone phosphate (DHAP) and lactaldehyde<ref>PMID:10821675</ref>.<br /> | '''Fuculose-1-phosphate aldolase''' catalyzes the cleavage of fuculose-1-phosphate to dihydroxyacetone phosphate (DHAP) and lactaldehyde<ref>PMID:10821675</ref>.<br /> | ||
'''HpcH/HpaI aldolase''' catalyzes the conversion of 4-hydroxy-2-oxo-heptane-1,7-dioate into pyruvate and succinate. It is part of the aromatic compounds degradation<ref>PMID:17881002</ref>.<br /> | '''HpcH/HpaI aldolase''' catalyzes the conversion of 4-hydroxy-2-oxo-heptane-1,7-dioate into pyruvate and succinate. It is part of the aromatic compounds degradation<ref>PMID:17881002</ref>.<br /> | ||
| Line 51: | Line 52: | ||
The regulation of fructose 1,6-bisphosphate aldolase is not well understood, but the understanding is every-increasing. As it is currently observed, aldolase C appears to be regulated mainly by the gene expression--the concentration of mRNA in the cytoplasm.<ref>Paolella, G, Buono, P, Mancini, F P, Izzo, P, and Salvatore, F. "Structure and expression of mouse aldolase genes." Eur. J. Biochem.. 156. (1986): 229-235.</ref> It is also known that adenosine 3',5'-cyclicmonophosphate (cAMP) affects the expression of the gene. cAMP concentration has been positively correlated with aldolase C expression. It is believed that cAMP acts upon a section of the promotor region, distal element D, causing the transcriptional promoter, NGFI-B, to bind. Once bound, the promoter activates the transcription of the gene coding for fructose bisphosphate aldolase.<ref>Buono, P, Cassano, S, Alfieri, A, Mancini, A, and Salvatore, F. "Human aldolase C gene expression is regulated by adenosine 30,50-cyclic monophosphate (cAMP) in PC12 cells." Gene. 291. (2002): 115-121.</ref> Given the inhibitory effects of an oxidant in the presence of aldolase, it is possible that this could be a mechanism of regulation of the enzyme. The deactivation that accompanies the oxidation of the surface thiol of Cys72 could be used intracellularly to slow the catalysis of the enzyme and regulate glycolysis.<ref name="kinetics" /> | The regulation of fructose 1,6-bisphosphate aldolase is not well understood, but the understanding is every-increasing. As it is currently observed, aldolase C appears to be regulated mainly by the gene expression--the concentration of mRNA in the cytoplasm.<ref>Paolella, G, Buono, P, Mancini, F P, Izzo, P, and Salvatore, F. "Structure and expression of mouse aldolase genes." Eur. J. Biochem.. 156. (1986): 229-235.</ref> It is also known that adenosine 3',5'-cyclicmonophosphate (cAMP) affects the expression of the gene. cAMP concentration has been positively correlated with aldolase C expression. It is believed that cAMP acts upon a section of the promotor region, distal element D, causing the transcriptional promoter, NGFI-B, to bind. Once bound, the promoter activates the transcription of the gene coding for fructose bisphosphate aldolase.<ref>Buono, P, Cassano, S, Alfieri, A, Mancini, A, and Salvatore, F. "Human aldolase C gene expression is regulated by adenosine 30,50-cyclic monophosphate (cAMP) in PC12 cells." Gene. 291. (2002): 115-121.</ref> Given the inhibitory effects of an oxidant in the presence of aldolase, it is possible that this could be a mechanism of regulation of the enzyme. The deactivation that accompanies the oxidation of the surface thiol of Cys72 could be used intracellularly to slow the catalysis of the enzyme and regulate glycolysis.<ref name="kinetics" /> | ||
| + | |||
| + | ==3D structures of aldolase== | ||
| + | [[Aldolase 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
=3D structures of Aldolase= | =3D structures of Aldolase= | ||
| Line 83: | Line 88: | ||
**[[4moz]] – FBPA – ''Slackia heliotrinireducens''<br /> | **[[4moz]] – FBPA – ''Slackia heliotrinireducens''<br /> | ||
**[[4d2j]], [[4tu1]], [[5tjs]] – TgFBPA – ''Toxoplasma gondii''<br /> | **[[4d2j]], [[4tu1]], [[5tjs]] – TgFBPA – ''Toxoplasma gondii''<br /> | ||
| + | **[[5u4n]] – FBPA – ''Neisseria gonorrhoeae''<br /> | ||
*FBPA complex | *FBPA complex | ||
| Line 115: | Line 121: | ||
**[[1rv8]], [[1rvg]] – FBPA + metal – ''Thermus aquaticus''<br /> | **[[1rv8]], [[1rvg]] – FBPA + metal – ''Thermus aquaticus''<br /> | ||
**[[1b57]] – EcFBPA + oxamate<br /> | **[[1b57]] – EcFBPA + oxamate<br /> | ||
| - | **[[3t2c]] – TnFBPA + | + | **[[3t2c]] – TnFBPA + DHAP<br /> |
| - | **[[3t2g]] – TnFBPA (mutant) + | + | **[[3t2g]] – TnFBPA (mutant) + DHAP<br /> |
**[[3t2d]] – TnFBPA + FBP<br /> | **[[3t2d]] – TnFBPA + FBP<br /> | ||
**[[3t2e]] – TnFBPA + F6P<br /> | **[[3t2e]] – TnFBPA + F6P<br /> | ||
| - | **[[3t2f]] – TnFBPA + EDTA + | + | **[[3t2f]] – TnFBPA + EDTA + DHAP<br /> |
| - | **[[5tk3]], [[5tkc]] – TgFBPA + G3P + | + | **[[5tk3]], [[5tkc]] – TgFBPA + G3P + DHAP <br /> |
**[[5tkn]], [[5tkp]] – TgFBPA + P6F <br /> | **[[5tkn]], [[5tkp]] – TgFBPA + P6F <br /> | ||
| - | **[[5tkl]] – TgFBPA + P6F + | + | **[[5tkl]] – TgFBPA + P6F + DHAP <br /> |
*Fructose–6-phosphate aldolase | *Fructose–6-phosphate aldolase | ||
| Line 201: | Line 207: | ||
**[[1eun]], [[1fq0]] – EcKDPG<br /> | **[[1eun]], [[1fq0]] – EcKDPG<br /> | ||
**[[1fwr]] - EcKDPG (mutant)<br /> | **[[1fwr]] - EcKDPG (mutant)<br /> | ||
| + | **[[5xse]] - ZmKDPG – ''Zymomonas mobilis''<br /> | ||
*KDPG complex | *KDPG complex | ||
| Line 206: | Line 213: | ||
**[[1wa3]] - TmKDPG + pyruvate <br /> | **[[1wa3]] - TmKDPG + pyruvate <br /> | ||
**[[1eua]] - EcKDPG + pyruvate <br /> | **[[1eua]] - EcKDPG + pyruvate <br /> | ||
| + | **[[5xsf]] - ZmKDPG + pyruvate <br /> | ||
*keto-deoxygluconate aldolase (KDG) | *keto-deoxygluconate aldolase (KDG) | ||
| Line 232: | Line 240: | ||
**[[1dxe]] – EcDGA<br /> | **[[1dxe]] – EcDGA<br /> | ||
**[[1dxf]] – EcDGA + pyruvate <br /> | **[[1dxf]] – EcDGA + pyruvate <br /> | ||
| + | |||
| + | *2,4-dihydroxyhept-2-ene-1,7-dioic acid aldolase | ||
| + | |||
| + | **[[6bdd]] – KaALD + heme – ''Kordia algicida''<br /> | ||
| + | **[[6bde]] – KaALD (mutant) + heme <br /> | ||
*Phospho-2-dehydro-3-deoxyheptonate aldolase | *Phospho-2-dehydro-3-deoxyheptonate aldolase | ||
| Line 251: | Line 264: | ||
**[[4to8]] – SsFBPA <br /> | **[[4to8]] – SsFBPA <br /> | ||
**[[5u7s]] – ALD – ''Acinetobacter baumannii''<br /> | **[[5u7s]] – ALD – ''Acinetobacter baumannii''<br /> | ||
| + | **[[5vjd]] – EcALDII + DHAP + Zn<br /> | ||
| + | **[[5vje]] – EcALDII + glucitol bisphosphate + Zn<br /> | ||
| + | **[[5vjf]], [[5uck]] – HpALDII + DHAP + Zn<br /> | ||
| + | **[[5ucn]], [[5ucp]], [[5ucz]], [[5ud0]], [[5ud2]] – HpALDII (mutant) + DHAP + Zn<br /> | ||
| + | **[[5ucs]], [[5ud1]] – HpALDII (mutant) + Zn<br /> | ||
| + | **[[5ud3]] – HpALDII (mutant) + diphosphono-fructose + Zn<br /> | ||
| + | **[[5ud4]] – HpALDII (mutant) + diphosphono-tagatose + Zn<br /> | ||
*Tagatose–1,6-bisphosphate aldolase | *Tagatose–1,6-bisphosphate aldolase | ||
| Line 276: | Line 296: | ||
**[[4c24]] ,[[4c25]] – SpFPA - ''Streptococcus pneumoniae''<br /> | **[[4c24]] ,[[4c25]] – SpFPA - ''Streptococcus pneumoniae''<br /> | ||
**[[4xxf]] – FPA – ''Glaciozyma antarctica''<br /> | **[[4xxf]] – FPA – ''Glaciozyma antarctica''<br /> | ||
| + | **[[6btd]] – BtFPA – ''Bacillus thuringiensis''<br /> | ||
| + | **[[6btg]] – BtFPA + DHAP <br /> | ||
*Sphingosin-1-phosphate aldolase | *Sphingosin-1-phosphate aldolase | ||
| Line 307: | Line 329: | ||
**[[3wgc]] – AjThrA (mutant)<br /> | **[[3wgc]] – AjThrA (mutant)<br /> | ||
**[[4v15]] – ThrA – ''Achromobacter xylosoxidans''<br /> | **[[4v15]] – ThrA – ''Achromobacter xylosoxidans''<br /> | ||
| + | **[[5vye]] – PpThrA + pyridoxime derivative<br /> | ||
*Phenylserine aldolase | *Phenylserine aldolase | ||
Revision as of 09:58, 5 March 2019
| |||||||||||
3D structures of Aldolase
Updated on 05-March-2019
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ Schurmann M, Sprenger GA. Fructose-6-phosphate aldolase is a novel class I aldolase from Escherichia coli and is related to a novel group of bacterial transaldolases. J Biol Chem. 2001 Apr 6;276(14):11055-61. Epub 2000 Dec 18. PMID:11120740 doi:http://dx.doi.org/10.1074/jbc.M008061200
- ↑ Salleron L, Magistrelli G, Mary C, Fischer N, Bairoch A, Lane L. DERA is the human deoxyribose phosphate aldolase and is involved in stress response. Biochim Biophys Acta. 2014 Dec;1843(12):2913-25. doi:, 10.1016/j.bbamcr.2014.09.007. Epub 2014 Sep 16. PMID:25229427 doi:http://dx.doi.org/10.1016/j.bbamcr.2014.09.007
- ↑ Goyer A, Illarionova V, Roje S, Fischer M, Bacher A, Hanson AD. Folate biosynthesis in higher plants. cDNA cloning, heterologous expression, and characterization of dihydroneopterin aldolases. Plant Physiol. 2004 May;135(1):103-11. Epub 2004 Apr 23. PMID:15107504 doi:http://dx.doi.org/10.1104/pp.103.038430
- ↑ Smith BJ, Lawrence MC, Barbosa JA. Substrate-Assisted Catalysis in Sialic Acid Aldolase. J Org Chem. 1999 Feb 5;64(3):945-949. PMID:11674166
- ↑ Wang W, Mazurkewich S, Kimber MS, Seah SY. Structural and kinetic characterization of 4-hydroxy-4-methyl-2-oxoglutarate (HMG)/4-carboxy-4-hydroxy-2-oxoadipate (CHA) aldolase: a protocatechuate degradation enzyme evolutionarily convergent with the HpaI and DmpG pyruvate aldolases. J Biol Chem. 2010 Sep 15. PMID:20843800 doi:10.1074/jbc.M110.159509
- ↑ Powlowski J, Sahlman L, Shingler V. Purification and properties of the physically associated meta-cleavage pathway enzymes 4-hydroxy-2-ketovalerate aldolase and aldehyde dehydrogenase (acylating) from Pseudomonas sp. strain CF600. J Bacteriol. 1993 Jan;175(2):377-85. PMID:8419288
- ↑ Bell BJ, Watanabe L, Rios-Steiner JL, Tulinsky A, Lebioda L, Arni RK. Structure of 2-keto-3-deoxy-6-phosphogluconate (KDPG) aldolase from Pseudomonas putida. Acta Crystallogr D Biol Crystallogr. 2003 Aug;59(Pt 8):1454-8. Epub 2003, Jul 23. PMID:12876349
- ↑ Zgiby SM, Thomson GJ, Qamar S, Berry A. Exploring substrate binding and discrimination in fructose1, 6-bisphosphate and tagatose 1,6-bisphosphate aldolases. Eur J Biochem. 2000 Mar;267(6):1858-68. PMID:10712619
- ↑ Hall DR, Bond CS, Leonard GA, Watt CI, Berry A, Hunter WN. Structure of tagatose-1,6-bisphosphate aldolase. Insight into chiral discrimination, mechanism, and specificity of class II aldolases. J Biol Chem. 2002 Jun 14;277(24):22018-24. Epub 2002 Apr 8. PMID:11940603 doi:http://dx.doi.org/10.1074/jbc.M202464200
- ↑ Joerger AC, Gosse C, Fessner WD, Schulz GE. Catalytic action of fuculose 1-phosphate aldolase (class II) as derived from structure-directed mutagenesis. Biochemistry. 2000 May 23;39(20):6033-41. PMID:10821675
- ↑ Rea D, Fulop V, Bugg TD, Roper DI. Structure and mechanism of HpcH: a metal ion dependent class II aldolase from the homoprotocatechuate degradation pathway of Escherichia coli. J Mol Biol. 2007 Nov 2;373(4):866-76. Epub 2007 Jun 26. PMID:17881002 doi:10.1016/j.jmb.2007.06.048
- ↑ Riedel TJ, Johnson LC, Knight J, Hantgan RR, Holmes RP, Lowther WT. Structural and Biochemical Studies of Human 4-hydroxy-2-oxoglutarate Aldolase: Implications for Hydroxyproline Metabolism in Primary Hyperoxaluria. PLoS One. 2011;6(10):e26021. Epub 2011 Oct 6. PMID:21998747 doi:10.1371/journal.pone.0026021
- ↑ KARASEK MA, GREENBERG DM. Studies on the properties of threonine aldolases. J Biol Chem. 1957 Jul;227(1):191-205. PMID:13449064
- ↑ 14.0 14.1 14.2 Voet, D, Voet, J, & Pratt, C. (2008). Fundamentals of biochemistry, third edition. Hoboken, NJ: Wiley & Sons, Inc.
- ↑ Protein: fructose-1,6-bisphosphate aldolase from human (homo sapiens), muscle isozyme. (2009). Retrieved from http://scop.mrc-lmb.cam.ac.uk
- ↑ 16.0 16.1 16.2 Gefflaut, T., B. Casimir, J. Perie, and M. Willson. "Class I Aldolases: Substrate Specificity, Mechanism, Inhibitors and Structural Aspects." Prog. Biophys. molec. Biol.. 63. (1995): 301-340.
- ↑ Dalby A, Dauter Z, Littlechild JA. Crystal structure of human muscle aldolase complexed with fructose 1,6-bisphosphate: mechanistic implications. Protein Sci. 1999 Feb;8(2):291-7. PMID:10048322
- ↑ 18.0 18.1 Sygusch, J., and Beaudry, D. "Allosteric communication in mammalian muscle aldolase." Biochem. J.. 327. (1997): 717-720.
- ↑ Paolella, G, Buono, P, Mancini, F P, Izzo, P, and Salvatore, F. "Structure and expression of mouse aldolase genes." Eur. J. Biochem.. 156. (1986): 229-235.
- ↑ Buono, P, Cassano, S, Alfieri, A, Mancini, A, and Salvatore, F. "Human aldolase C gene expression is regulated by adenosine 30,50-cyclic monophosphate (cAMP) in PC12 cells." Gene. 291. (2002): 115-121.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Sophie Mullinix, Jaime Prilusky, Austin Drake, David Canner