Antizyme Inhibitor
From Proteopedia
(Difference between revisions)
m |
|||
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
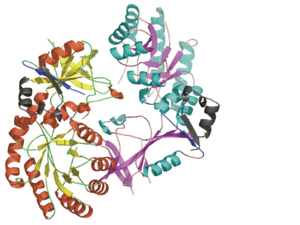
<StructureSection load="" size="400" color="white" caption="Mouse antizyme inhibitor 1 dimer [[3btn]]" scene='Antizyme_Inhibitor/Azi/1' spinBox="true" > | <StructureSection load="" size="400" color="white" caption="Mouse antizyme inhibitor 1 dimer [[3btn]]" scene='Antizyme_Inhibitor/Azi/1' spinBox="true" > | ||
[[Image:AziFig.PNG|left|300px]] | [[Image:AziFig.PNG|left|300px]] | ||
| - | == | + | ==Antizyme Inhibitor== |
{{ABSTRACT_PUBMED_18369191}} | {{ABSTRACT_PUBMED_18369191}} | ||
{{Clear}} | {{Clear}} | ||
| - | + | Each <scene name='Antizyme_Inhibitor/Monomer/5'>monomer</scene> consists of two domains: a <scene name='Antizyme_Inhibitor/Monomer/6'>TIM-like</scene> α/β-barrel [http://en.wikipedia.org/wiki/TIM_barrel] domain (residues 45–280) and a modified <scene name='Antizyme_Inhibitor/Monomer/7'>Greek key</scene> [http://en.wikipedia.org/wiki/Greek_key] β-sheet domain (residues 8–44 and 281–435). <font color='red'><b>Helices</b></font> [http://en.wikipedia.org/wiki/Alpha_helix] are colored in <font color='red'><b>red</b></font> and <font color='black'><b>β sheets</b></font> [http://en.wikipedia.org/wiki/Beta_sheet] in <font color='black'><b>yellow</b></font>. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
{{Clear}} | {{Clear}} | ||
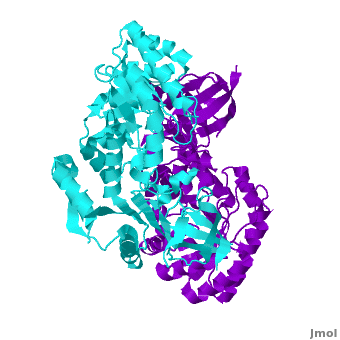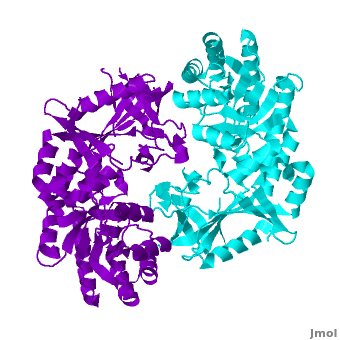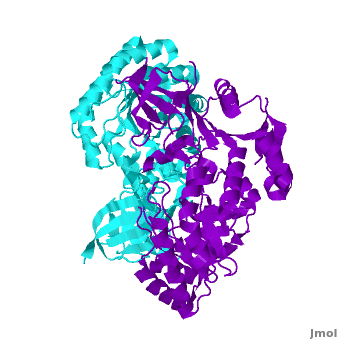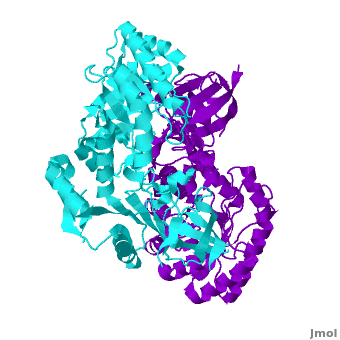
A sequence alignment and structural comparison of mouse AzI crystallographic dimer to mouse, human, and [http://en.wikipedia.org/wiki/Trypanosome trypanosome] [http://en.wikipedia.org/wiki/Ornithine_decarboxylase ODC] (mODC, hODC, and tODC, respectively) show high sequence identity (~50%) and structural similarity between AzI and ODC monomers (RMSD values of 1.85 Å, 1.6 Å, and 1.5 Å, respectively). The <scene name='Antizyme_Inhibitor/Azi_odc/10'>structural comparison</scene> of mouse AzI crystallographic dimer (mAzI, <font color='cyan'><b>cyan</b></font> and <font color='blueviolet'><b>blueviolet</b></font>) to mODC (PDB code [[7odc]], (<font color='red'><b>red</b></font> and <font color='lime'><b>lime</b></font>) is shown. Superposition of the <scene name='Antizyme_Inhibitor/Azi_odc/11'>interface</scene> of mAzI and mODC showing the inter-subunit variable loops (AzI residues 355–362 and 387–401). <font color='black'><b>AzI loops</b></font> are in <font color='black'><b>black</b></font>, and <font color='black'><b>ODC loops</b></font> are in <font color='black'><b>yellow</b></font>. | A sequence alignment and structural comparison of mouse AzI crystallographic dimer to mouse, human, and [http://en.wikipedia.org/wiki/Trypanosome trypanosome] [http://en.wikipedia.org/wiki/Ornithine_decarboxylase ODC] (mODC, hODC, and tODC, respectively) show high sequence identity (~50%) and structural similarity between AzI and ODC monomers (RMSD values of 1.85 Å, 1.6 Å, and 1.5 Å, respectively). The <scene name='Antizyme_Inhibitor/Azi_odc/10'>structural comparison</scene> of mouse AzI crystallographic dimer (mAzI, <font color='cyan'><b>cyan</b></font> and <font color='blueviolet'><b>blueviolet</b></font>) to mODC (PDB code [[7odc]], (<font color='red'><b>red</b></font> and <font color='lime'><b>lime</b></font>) is shown. Superposition of the <scene name='Antizyme_Inhibitor/Azi_odc/11'>interface</scene> of mAzI and mODC showing the inter-subunit variable loops (AzI residues 355–362 and 387–401). <font color='black'><b>AzI loops</b></font> are in <font color='black'><b>black</b></font>, and <font color='black'><b>ODC loops</b></font> are in <font color='black'><b>yellow</b></font>. | ||
| Line 16: | Line 12: | ||
<scene name='Antizyme_Inhibitor/Binding_site/6'>Overlap</scene> of the AzI and mODC structures suggests that AzI does not bind [http://en.wikipedia.org/wiki/Pyridoxal_phosphate PLP]. <font color='black'><b>PLP</b></font> is in <font color='black'><b>yellow</b></font>, <font color='lime'><b>ODC residues D88, R154, R277, and Y389 are in lime</b></font>, and the corresponding <font color='magenta'><b>AzI residues A88, H154, S274, and D387 are in magenta</b></font>. Many of the residues participating in PLP-ODC binding are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbers follow the sequence of AzI). Notably, the absence of even one of these interactions, (''e.g.'' ODC R277A mutant) results in a 100-fold decrease in PLP binding, a 50% drop in K<sub>cat</sub>, and a 7-fold decrease in K<sub>M</sub>. | <scene name='Antizyme_Inhibitor/Binding_site/6'>Overlap</scene> of the AzI and mODC structures suggests that AzI does not bind [http://en.wikipedia.org/wiki/Pyridoxal_phosphate PLP]. <font color='black'><b>PLP</b></font> is in <font color='black'><b>yellow</b></font>, <font color='lime'><b>ODC residues D88, R154, R277, and Y389 are in lime</b></font>, and the corresponding <font color='magenta'><b>AzI residues A88, H154, S274, and D387 are in magenta</b></font>. Many of the residues participating in PLP-ODC binding are not conserved in AzI. These include D88A, R154H, R277S, D332E, and Y389D (ODC residue numbers follow the sequence of AzI). Notably, the absence of even one of these interactions, (''e.g.'' ODC R277A mutant) results in a 100-fold decrease in PLP binding, a 50% drop in K<sub>cat</sub>, and a 7-fold decrease in K<sub>M</sub>. | ||
| - | </StructureSection> | ||
==3D structures of Antizyme Inhibitor== | ==3D structures of Antizyme Inhibitor== | ||
| + | [[Antizyme inhibitor 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Additional Resources== | ==Additional Resources== | ||
Current revision
| |||||||||||
Additional Resources
For additional information, see: Cancer
Reference
Shira Albeck, Orly Dym, Tamar Unger, Zohar Snapir, Zippy Bercovich and Chaim Kahana. Crystallographic and biochemical studies revealing the structural basis for antizyme inhibitor function. Protein Sci. 2008 May; 17(5): 793-802. Epub 2008 Mar 27.
Proteopedia Page Contributors and Editors (what is this?)
Alexander Berchansky, Michal Harel, Joel L. Sussman, Dinesh Kulhary, David Canner, Jaime Prilusky, Orly Dym