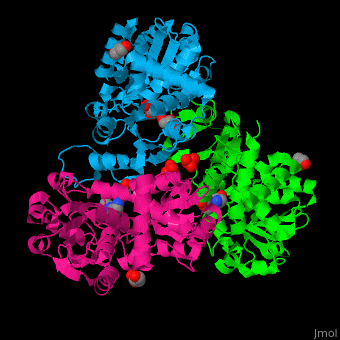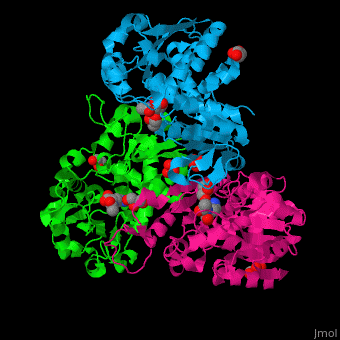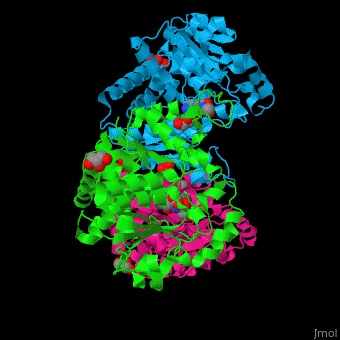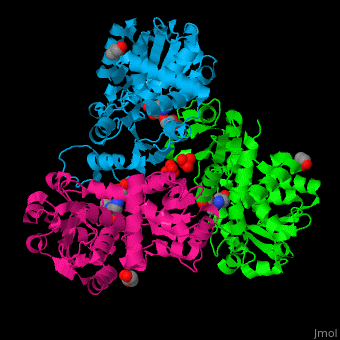
N-acetylornithine carbamoyltransferase
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='450' side='right' caption='N-acetylornithine carbamoyltransferase complex with acetylornithine, glycerol and sulfate (PDB code [[3kzn]]) ' scene='75/750306/Cv/3' pspeed='8'> |
== Function == | == Function == | ||
'''N-acetylornithine carbamoyltransferase''' (AOCT) catalyzes the reversible conversion of carbamoyl phosphate and N-acetyl-L-ornithine into N-acetyl-L-citrulline and phosphate. | '''N-acetylornithine carbamoyltransferase''' (AOCT) catalyzes the reversible conversion of carbamoyl phosphate and N-acetyl-L-ornithine into N-acetyl-L-citrulline and phosphate. | ||
| Line 5: | Line 5: | ||
== Structural highlights == | == Structural highlights == | ||
| - | AOCT motif HCLP interacts with the produce acetyl citrulline. The <scene name='75/750306/Cv/ | + | AOCT motif HCLP interacts with the produce acetyl citrulline. The <scene name='75/750306/Cv/7'>substrate acetyl ornithine makes numerous H-bonds with residues in the active site of AOCT</scene><ref>PMID:16741992</ref>. Water molecules are shown as red spheres. |
</StructureSection> | </StructureSection> | ||
==3D structures of N-acetylornithine carbamoyltransferase== | ==3D structures of N-acetylornithine carbamoyltransferase== | ||
| Line 16: | Line 16: | ||
[[3kzm]] - XcAOCT + carbamylphosphate<br /> | [[3kzm]] - XcAOCT + carbamylphosphate<br /> | ||
[[3kzn]] - XcAOCT + acetyl ornithine<br /> | [[3kzn]] - XcAOCT + acetyl ornithine<br /> | ||
| + | [[3kzk]] - XcAOCT + acetyl citrulline<br /> | ||
[[3kzo]] - XcAOCT + acetyl norvaline + carbamyl phosphate<br /> | [[3kzo]] - XcAOCT + acetyl norvaline + carbamyl phosphate<br /> | ||
[[3l02]], [[3l04]], [[3l05]], [[3l06]] - XcAOCT (mutant) + norvaline derivative + carbamyl phosphate<br /> | [[3l02]], [[3l04]], [[3l05]], [[3l06]] - XcAOCT (mutant) + norvaline derivative + carbamyl phosphate<br /> | ||
| Line 21: | Line 22: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D structures of N-acetylornithine carbamoyltransferase
Updated on 21-July-2019
3m4j - XcAOCT + acetyl-phosphonoacetyl ornithine - Xanthomonas campestris
3m4n, 3m5c, 3m5d - XcAOCT (mutant) + acetyl-phophonoacetyl ornithine
3kzc - XcAOCT
3kzm - XcAOCT + carbamylphosphate
3kzn - XcAOCT + acetyl ornithine
3kzk - XcAOCT + acetyl citrulline
3kzo - XcAOCT + acetyl norvaline + carbamyl phosphate
3l02, 3l04, 3l05, 3l06 - XcAOCT (mutant) + norvaline derivative + carbamyl phosphate
References
- ↑ Shi D, Yu X, Roth L, Morizono H, Tuchman M, Allewell NM. Structures of N-acetylornithine transcarbamoylase from Xanthomonas campestris complexed with substrates and substrate analogs imply mechanisms for substrate binding and catalysis. Proteins. 2006 Aug 1;64(2):532-42. PMID:16741992 doi:10.1002/prot.21013