Histone deacetylase
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
== Structural highlights == | == Structural highlights == | ||
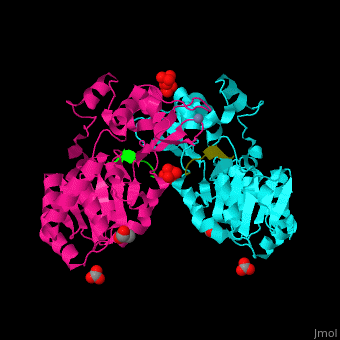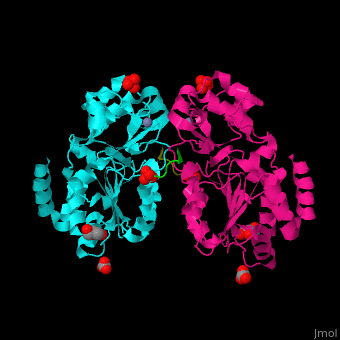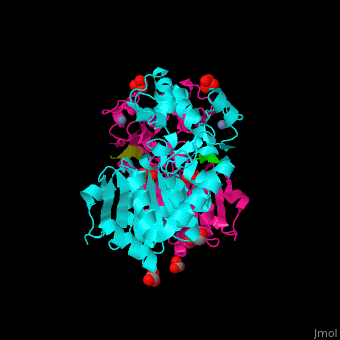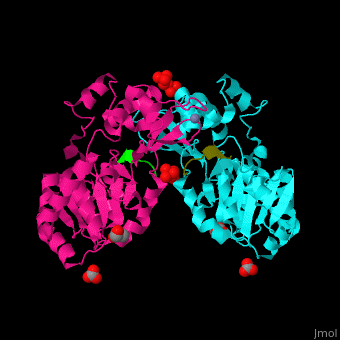
The biological assembly of Human sirtuin-3 is <scene name='48/483191/Cv/5'>homodimer</scene>. The <scene name='48/483191/Cv/6'>acetylated lysine of the peptide is inserted in an extended conformation</scene> between the 2 domains of SIRT3 - a large Rossman fold, NAD-binding domain and a small Zn-binding one)<ref>PMID:19535340</ref>. Water molecule shown as red sphere. | The biological assembly of Human sirtuin-3 is <scene name='48/483191/Cv/5'>homodimer</scene>. The <scene name='48/483191/Cv/6'>acetylated lysine of the peptide is inserted in an extended conformation</scene> between the 2 domains of SIRT3 - a large Rossman fold, NAD-binding domain and a small Zn-binding one)<ref>PMID:19535340</ref>. Water molecule shown as red sphere. | ||
| + | |||
| + | == 3D Structures of histone deacetylase == | ||
| + | [[Histone deacetylase 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
== 3D Structures of histone deacetylase == | == 3D Structures of histone deacetylase == | ||
| Line 132: | Line 136: | ||
**[[4iao]] – ySIRT2 + SIRT4 + ADP-ribose + Zn<br /> | **[[4iao]] – ySIRT2 + SIRT4 + ADP-ribose + Zn<br /> | ||
**[[1j8f]], [[3zgo]] – hSIRT2 + Zn<br /> | **[[1j8f]], [[3zgo]] – hSIRT2 + Zn<br /> | ||
| + | **[[5yqo]], [[5yqn]], [[5yqm]], [[5yql]] – hSIRT2 + inhibitor + Zn<br /> | ||
| + | **[[5y0z]] – hSIRT2 (mutant) + inhibitor + Zn<br /> | ||
**[[3zgv]], [[5d7o]] – hSIRT2+ ADP ribose + Zn<br /> | **[[3zgv]], [[5d7o]] – hSIRT2+ ADP ribose + Zn<br /> | ||
**[[4y6q]] – hSIRT2+ ADP ribose derivative + Zn<br /> | **[[4y6q]] – hSIRT2+ ADP ribose derivative + Zn<br /> | ||
| Line 158: | Line 164: | ||
**[[4fz3]] – hSIRT3 + acetyl lysine peptide + coumarine derivative + Zn<br /> | **[[4fz3]] – hSIRT3 + acetyl lysine peptide + coumarine derivative + Zn<br /> | ||
**[[4c78]], [[4c7b]] – hSIRT3 + acetyl lysine peptide + resveratol derivative + Zn<br /> | **[[4c78]], [[4c7b]] – hSIRT3 + acetyl lysine peptide + resveratol derivative + Zn<br /> | ||
| + | **[[5zgc]], [[5z94]], [[5z93]] – hSIRT3 + histone peptide + Zn<br /> | ||
**[[4jsr]], [[4jt8]], [[4jt9]], [[4o8z]] – hSIRT3 + inhibitor + Zn<br /> | **[[4jsr]], [[4jt8]], [[4jt9]], [[4o8z]] – hSIRT3 + inhibitor + Zn<br /> | ||
**[[4gbn5]] – hSIRT3 + inhibitor + carba-NAD + Zn<br/ > | **[[4gbn5]] – hSIRT3 + inhibitor + carba-NAD + Zn<br/ > | ||
| Line 177: | Line 184: | ||
**[[4f56]] – hSIRT5 + peptide + intermediate + Zn<br /> | **[[4f56]] – hSIRT5 + peptide + intermediate + Zn<br /> | ||
**[[4hda]] – hSIRT5 + acetyl lysine peptide + resveratrol + Zn<br /> | **[[4hda]] – hSIRT5 + acetyl lysine peptide + resveratrol + Zn<br /> | ||
| + | **[[6acp]], [[6aco]], [[6acl]], [[6ace]] – hSIRT5 + succinyl peptide + Zn<br /> | ||
**[[4utn]], [[4utr]], [[4utv]], [[4utx]], [[4utz]], [[4uu7]], [[4uu8]], [[4uua]], [[4uub]], [[6flg]], [[6fkz]], [[6fky]] – zSIRT5 + acetyl lysine peptide + inhibitor + Zn <br /> | **[[4utn]], [[4utr]], [[4utv]], [[4utx]], [[4utz]], [[4uu7]], [[4uu8]], [[4uua]], [[4uub]], [[6flg]], [[6fkz]], [[6fky]] – zSIRT5 + acetyl lysine peptide + inhibitor + Zn <br /> | ||
**[[6enx]], [[6eo0]], [[6eqs]] – zSIRT5 + inhibitor + Zn <br /> | **[[6enx]], [[6eo0]], [[6eqs]] – zSIRT5 + inhibitor + Zn <br /> | ||
| Line 184: | Line 192: | ||
**[[3k35]] – hSIRT6 + ADP-ribose + Zn<br /> | **[[3k35]] – hSIRT6 + ADP-ribose + Zn<br /> | ||
| + | **[[5x16]] – hSIRT6 + oxolan derivative + Zn<br /> | ||
| + | **[[6hoy]] – hSIRT6 + ADP-ribose + trichostatin + Zn<br /> | ||
**[[3pki]], [[3pkj]] – hSIRT6 (mutant) + ADP-ribose derivative + Zn<br /> | **[[3pki]], [[3pkj]] – hSIRT6 (mutant) + ADP-ribose derivative + Zn<br /> | ||
**[[3zg6]] – hSIRT6 + acetyl lysine peptide + ADP-ribose + Zn<br /> | **[[3zg6]] – hSIRT6 + acetyl lysine peptide + ADP-ribose + Zn<br /> | ||
**[[5mgn]], [[5mfz]], [[5mfp]], [[5mf6]] – hSIRT6 + activator + Zn<br /> | **[[5mgn]], [[5mfz]], [[5mfp]], [[5mf6]] – hSIRT6 + activator + Zn<br /> | ||
| + | **[[5y2f]] – hSIRT6 + peptide activator + Zn<br /> | ||
*SIRT7 | *SIRT7 | ||
Revision as of 09:09, 31 July 2019
| |||||||||||
3D Structures of histone deacetylase
Updated on 31-July-2019
References
- ↑ Joshi P, Greco TM, Guise AJ, Luo Y, Yu F, Nesvizhskii AI, Cristea IM. The functional interactome landscape of the human histone deacetylase family. Mol Syst Biol. 2013;9:672. doi: 10.1038/msb.2013.26. PMID:23752268 doi:http://dx.doi.org/10.1038/msb.2013.26
- ↑ Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 2008 Feb;7(2):104-12. doi: 10.1016/j.cmet.2007.11.006. PMID:18249170 doi:http://dx.doi.org/10.1016/j.cmet.2007.11.006
- ↑ Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001 Dec;1(3):194-202. PMID:11902574 doi:http://dx.doi.org/10.1038/35106079
- ↑ Yamamoto H, Schoonjans K, Auwerx J. Sirtuin functions in health and disease. Mol Endocrinol. 2007 Aug;21(8):1745-55. Epub 2007 Apr 24. PMID:17456799 doi:http://dx.doi.org/10.1210/me.2007-0079
- ↑ Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007 Oct;12(10):1247-52. PMID:17962618 doi:http://dx.doi.org/10.1634/theoncologist.12-10-1247
- ↑ Jin L, Wei W, Jiang Y, Peng H, Cai J, Mao C, Dai H, Choy W, Bemis JE, Jirousek MR, Milne JC, Westphal CH, Perni RB. Crystal structures of human SIRT3 displaying substrate-induced conformational changes. J Biol Chem. 2009 Sep 4;284(36):24394-405. Epub 2009 Jun 16. PMID:19535340 doi:10.1074/jbc.M109.014928