We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Test180919
From Proteopedia
(Difference between revisions)
(New page: ==== Peptide bond ==== <applet load='180-180.pdb' size='400' frame='true' align='right' scene='User:Tilman_Schirmer/Sandbox_99/Diala/22'/> The <scene name='User:Tilman_Schirmer/Sandbox_...) |
|||
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='180-180.pdb' size='400' side='right' caption='' scene='User:Tilman_Schirmer/Sandbox_99/Diala/22'> | ||
==== Peptide bond ==== | ==== Peptide bond ==== | ||
| - | <applet load='180-180.pdb' size='400' frame='true' align='right' scene='User:Tilman_Schirmer/Sandbox_99/Diala/22'/> | ||
| - | |||
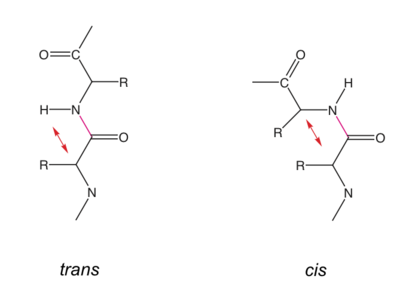
The <scene name='User:Tilman_Schirmer/Sandbox_99/Diala/22'>peptide bond </scene> (highlight in <scene name='User:Tilman_Schirmer/Sandbox_99/Toggle/1'>green</scene>) formation is a condensation reaction between the carboxyl group of the amino acid i and the amino group of the amino acid i+1. | The <scene name='User:Tilman_Schirmer/Sandbox_99/Diala/22'>peptide bond </scene> (highlight in <scene name='User:Tilman_Schirmer/Sandbox_99/Toggle/1'>green</scene>) formation is a condensation reaction between the carboxyl group of the amino acid i and the amino group of the amino acid i+1. | ||
| Line 20: | Line 19: | ||
==== Cis peptide bonds ==== | ==== Cis peptide bonds ==== | ||
| - | The ω torsion angle can adopt a value close to 0° (cis-conformation), when a Pro residue is the following residue (Xaa-Pro peptide bond). In this situation a <scene name=' | + | The ω torsion angle can adopt a value close to 0° (cis-conformation), when a Pro residue is the following residue (Xaa-Pro peptide bond). In this situation a <scene name='82/824563/Pro_213_in_cis_conformation/1'>cis-Pro</scene> and a <scene name='82/824563/Pro_211_in_trans_conformation/1'>trans-Pro</scene> are similarily unfavorable, since there is a steric clash between Cα,i with Cα,i+1 or Cδ,i+1, respectively. Conversely, the carbonyl O of residue i is in tight juxtaposition with Cδ,i+1 or Cα,i+1 (note that latter tight contact occurs in any trans peptide bond). |
| Line 36: | Line 35: | ||
Secondary structure of proteins http://proteopedia.org/wiki/index.php/User:Tilman_Schirmer/Sandbox_100 | Secondary structure of proteins http://proteopedia.org/wiki/index.php/User:Tilman_Schirmer/Sandbox_100 | ||
| + | </StructureSection> | ||
Current revision
| |||||||||||