Erythropoietin
From Proteopedia
(Difference between revisions)
| (11 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==Erythropoietin Structure, Function, and History== | ==Erythropoietin Structure, Function, and History== | ||
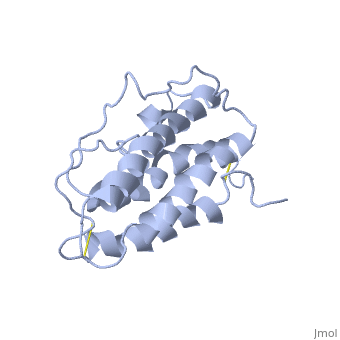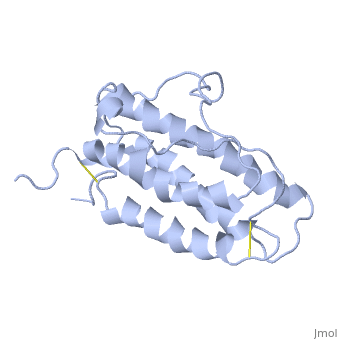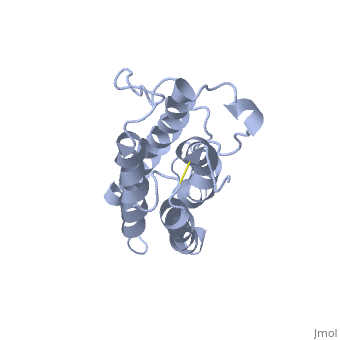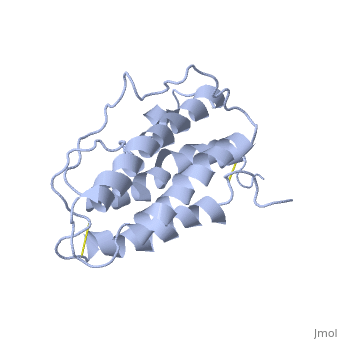
| - | <StructureSection load='1buy' size=' | + | <StructureSection load='1buy' size='350' side='right' caption='Human erythropoietin NMR structure (PDB code [[1buy]]).' scene=> |
| - | + | ==Introduction== | |
| - | + | ---- | |
| - | Erythropoietin (EPO) is a hormone produced in the kidneys that stimulates the formation of red blood cells. EPO is a glycoprotein that is stimulated when the levels of O2 are abnormally low. This event signals more red blood cells to made from the erythrocytes. Abnormal levels of erythropoietin can be associated with bone marrow disorders, kidney disease, or a synthesized recombinant form that has been injected into the blood stream. Synthesized recombinant EPO has made many headlines in the past few years, due to its use to by Tour de France athletes. They used EPO to illegally dope their blood and increase the amount of oxygen that can be consumed by the body at the time of administration thus increasing endurance. They used EPO because there was no test at the time that could differentiate between naturally produced EPO and the form that was injected. | + | '''Erythropoietin''' (EPO) is a [[Hormones|hormone]] produced in the kidneys that stimulates the formation of red blood cells. EPO is a glycoprotein that is stimulated when the levels of O2 are abnormally low. This event signals more red blood cells to made from the erythrocytes. Abnormal levels of erythropoietin can be associated with bone marrow disorders, kidney disease, or a synthesized recombinant form that has been injected into the blood stream. Synthesized recombinant EPO has made many headlines in the past few years, due to its use to by Tour de France athletes. They used EPO to illegally dope their blood and increase the amount of oxygen that can be consumed by the body at the time of administration thus increasing endurance. They used EPO because there was no test at the time that could differentiate between naturally produced EPO and the form that was injected. |
| - | + | ==History== | |
| + | |||
| + | ---- | ||
Miyake et al first purified a few milligrams of erythropoietin from 2500 L human urine in 1977 <ref>PMID: 6600633</ref>. This glycoprotein was first suspected as doctors observed anemia patients. Researchers noticed that patients with anemia had increased levels of erythropoiesis <ref>PMID:17253966</ref>. They noticed that the level of oxygen, seemed to be correlated with the amount of erythropoiesis, as well. After isolation, researchers worked to clone the gene so that they could produce the glycoprotein and treat hypoxia. The FDA approved this type of treatment in the 1990’s <ref>PMID:17253966</ref>. EPO works to stimulate the amount of red blood cells and the efficiency of hemoglobin, which increases oxygen library. This trait is what has led to many athletes to abuse this glycoprotein to improve their performance in their athletic sport, the most famous being Tour de France competitors (8). Most athletes involved in these scandal would intravenously inject themselves with this hormone (7). However, as medical techniques have advanced, some have started gene doping through in vivo and ex vivo gene transfer (7). | Miyake et al first purified a few milligrams of erythropoietin from 2500 L human urine in 1977 <ref>PMID: 6600633</ref>. This glycoprotein was first suspected as doctors observed anemia patients. Researchers noticed that patients with anemia had increased levels of erythropoiesis <ref>PMID:17253966</ref>. They noticed that the level of oxygen, seemed to be correlated with the amount of erythropoiesis, as well. After isolation, researchers worked to clone the gene so that they could produce the glycoprotein and treat hypoxia. The FDA approved this type of treatment in the 1990’s <ref>PMID:17253966</ref>. EPO works to stimulate the amount of red blood cells and the efficiency of hemoglobin, which increases oxygen library. This trait is what has led to many athletes to abuse this glycoprotein to improve their performance in their athletic sport, the most famous being Tour de France competitors (8). Most athletes involved in these scandal would intravenously inject themselves with this hormone (7). However, as medical techniques have advanced, some have started gene doping through in vivo and ex vivo gene transfer (7). | ||
| - | + | ==Structure== | |
| - | + | ---- | |
| - | ''' | + | The gene for EPO is found on chromosome 7 and is composed of five exons and four introns <ref>PMID: 1557429</ref>. The transcriptional product of this gene is an amino acid chain of 193 bases. During translation, the chain is modified to 166 amino acids <ref>PMID:17253966</ref>. The cleaved 27 amino acid leader sequence is made mostly of hydrophobic amino acids. After translation is complete, the C-terminus loses its final arginine residue to reach its final length of 165 amino acids residues <ref> Erslev, A. J., and J. Caro. "Physiologic and molecular biology of erythropoietin." Medical oncology and tumor pharmacotherapy 3.3-4 (1986): 159-164. </ref>. The total glycoprotein weighs 30 kDa with the peptide backbone weighing 18 kDa. EPO is a glycoprotein composed of only <scene name='12/128258/Epoalpha/1'>Alpha Helices</scene> <ref>Erslev, A. J., and J. Caro. "Physiologic and molecular biology of erythropoietin." Medical oncology and tumor pharmacotherapy 3.3-4 (1986): 159-164.</ref>. The sulfur of the cysteine residues links to form disulfide bonds. These disulfide bonds help keep EPO's structure. Helix A is connected to Helix D by <scene name='58/583377/Epocyslabels/1'>Cys7 and Cys161</scene>, while Helix A and Helix B are connected by <scene name='58/583377/Epocyslabels/1'>Cys29 and Cys33</scene> . EPO’s structure was determined in 1993. It is made up of four alpha helixes. EPO is produced mainly in the kidney, but further research has shown the brain and liver still produce small amounts <ref>Erslev, A. J., and J. Caro. "Physiologic and molecular biology of erythropoietin." Medical oncology and tumor pharmacotherapy 3.3-4 (1986): 159-164.</ref>. |
| - | + | ==Stimulus== | |
| - | + | ---- | |
| - | + | ||
| - | The | + | The levels of oxygen found in the blood moderate the process of erythropoiesis, which is the production of red blood cells. At low levels of oxygen, EPO released into the blood stream from the kidneys in adults humans. After release, the hormone travels to the bone marrow and binds to receptors, which starts the proliferation of red blood cells to increase the oxygen consumption. This increase in consumption will hopefully reestablish normal levels of blood in the system. |
| + | ==Receptors== | ||
| - | References | ||
---- | ---- | ||
| - | 1. Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology | + | The <scene name='12/128258/Eporeceptor/3'>EPO receptor</scene> of the blood marrow is part of the hematipoietic cytokine family. This receptor has a single transmembrane domain, that forms a homodimer complex until it is activated by the binding of EPO.This receptor is 484 amino acids long and weigh 52.6 kDa <ref>PMID:17253966</ref>. Once the homodimer is formed after the binding, autophosphorlation of the Jak2 kinases, which activates other cellular processes <ref>Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. This transmembrane receptor has two extracellular domains. This receptor has two disulfide bonds that are formed from 4 cystine residues, <scene name='58/583377/Eporeceptord1d2cyslabel/1'>Cys67 and Cys83 and Cys28 and Cys38</scene> <ref>Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology |
| - | and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23. | + | and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. The intracellular domain of this receptor does not possess any enzymatic activity like other receptors. When EPO comes in contact with the extracellular domains form a ligand bond. The extracellular sinding site 1 and Binding site 2 are composed of <scene name='58/583377/Eporeceptord1d2/1'>D1 and D2</scene> <ref>Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. When EPO binds, all loops on D1 and D2 of binding site one form a bind with EPO. However loop 4 of D1 on binding site 2 does not participate in the binding of EPO <ref>PMID: 9774108</ref>. After the biniding of EPO, 8 tyrosine residues are phosphoralated which activates the <scene name='58/583377/Jak2/2'>Jak2 kinase</scene> <ref>Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology |
| + | and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. This kinase helps regulate the transcription of different genes and expression of other proteins. | ||
| - | + | See also [[Erythropoietin receptor]] | |
| + | </StructureSection> | ||
| - | + | ==3D structures of erythropoietin== | |
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | + | [[1cn4]] – hEP (mutant) + EP receptor – human<br /> | |
| + | [[1buy]] – hEP (mutant) - NMR<br /> | ||
| + | [[1eer]] – hEP (mutant) + EP receptor (mutant)<br /> | ||
| - | + | ==References== | |
| - | + | ---- | |
| - | + | ||
| - | + | ||
| - | + | <references /> | |
| + | [[Category: Topic Page]] | ||
Current revision
Erythropoietin Structure, Function, and History
| |||||||||||
3D structures of erythropoietin
Updated on 11-December-2019
1cn4 – hEP (mutant) + EP receptor – human
1buy – hEP (mutant) - NMR
1eer – hEP (mutant) + EP receptor (mutant)
References
- ↑ Kawakita M, Ogawa M, Goldwasser E, Miyake T. Characterization of human megakaryocyte colony-stimulating factor in the urinary extracts from patients with aplastic anemia and idiopathic thrombocytopenic purpura. Blood. 1983 Mar;61(3):556-60. PMID:6600633
- ↑ Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007 Mar;78(3):183-205. Epub 2007 Jan 23. PMID:17253966 doi:http://dx.doi.org/10.1111/j.1600-0609.2007.00818.x
- ↑ Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007 Mar;78(3):183-205. Epub 2007 Jan 23. PMID:17253966 doi:http://dx.doi.org/10.1111/j.1600-0609.2007.00818.x
- ↑ Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992 Apr;72(2):449-89. PMID:1557429
- ↑ Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007 Mar;78(3):183-205. Epub 2007 Jan 23. PMID:17253966 doi:http://dx.doi.org/10.1111/j.1600-0609.2007.00818.x
- ↑ Erslev, A. J., and J. Caro. "Physiologic and molecular biology of erythropoietin." Medical oncology and tumor pharmacotherapy 3.3-4 (1986): 159-164.
- ↑ Erslev, A. J., and J. Caro. "Physiologic and molecular biology of erythropoietin." Medical oncology and tumor pharmacotherapy 3.3-4 (1986): 159-164.
- ↑ Erslev, A. J., and J. Caro. "Physiologic and molecular biology of erythropoietin." Medical oncology and tumor pharmacotherapy 3.3-4 (1986): 159-164.
- ↑ Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007 Mar;78(3):183-205. Epub 2007 Jan 23. PMID:17253966 doi:http://dx.doi.org/10.1111/j.1600-0609.2007.00818.x
- ↑ Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.
- ↑ Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.
- ↑ Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.
- ↑ Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998 Oct 1;395(6701):511-6. PMID:9774108 doi:http://dx.doi.org/10.1038/26773
- ↑ Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.