Journal:IUCrJ:S205225252000072X
From Proteopedia
(Difference between revisions)

| Line 1: | Line 1: | ||
| - | <StructureSection load='' size=' | + | <StructureSection load='' size='370' side='right' scene='83/834718/Cv/1' caption=''> |
===Comparing serial X-ray crystallography and microcrystal electron diffraction (MicroED) as methods for routine structure determination from small macromolecular crystals=== | ===Comparing serial X-ray crystallography and microcrystal electron diffraction (MicroED) as methods for routine structure determination from small macromolecular crystals=== | ||
<big>Alexander M Wolff, Iris D Young, Raymond G Sierra, Aaron S Brewster, Michael W Martynowycz, Eriko Nango, Michihiro Sugahara, Takanori Nakane, Kazutaka Ito, Andrew Aquila, Asmit Bhowmik, Justin T Biel, Sergio Carbajo, Aina E Cohen1; Saul Cortez, Ana Gonzalez, Tomoya Hino, Dohyun Im, Jake D Koralek, Minoru Kubo, Tomas S Lazarou, Takashi Nomura, Shigeki Owada, Avi J. Samelson, Tomoyuki Tanaka, Rie Tanaka, Erin M Thompson, Henry van den Bedem, Rahel A Woldeyes, Fumiaki Yumoto, Wei Zhao, Kensuke Tono, Sébastien Boutet, So Iwata, Tamir Gonen, Nicholas K Sauter, James S Fraser, Michael C Thompson</big> <ref>doi 10.1107/S205225252000072X</ref> | <big>Alexander M Wolff, Iris D Young, Raymond G Sierra, Aaron S Brewster, Michael W Martynowycz, Eriko Nango, Michihiro Sugahara, Takanori Nakane, Kazutaka Ito, Andrew Aquila, Asmit Bhowmik, Justin T Biel, Sergio Carbajo, Aina E Cohen1; Saul Cortez, Ana Gonzalez, Tomoya Hino, Dohyun Im, Jake D Koralek, Minoru Kubo, Tomas S Lazarou, Takashi Nomura, Shigeki Owada, Avi J. Samelson, Tomoyuki Tanaka, Rie Tanaka, Erin M Thompson, Henry van den Bedem, Rahel A Woldeyes, Fumiaki Yumoto, Wei Zhao, Kensuke Tono, Sébastien Boutet, So Iwata, Tamir Gonen, Nicholas K Sauter, James S Fraser, Michael C Thompson</big> <ref>doi 10.1107/S205225252000072X</ref> | ||
| Line 10: | Line 10: | ||
Our model system for this work was Cyclophilin A (CypA), which we had previously studied at multiple temperatures. As it turned out, both microED and XFELs enabled us to solve high-resolution structures of CypA, but the conformations of the protein were not the same. In the microED structure, CypA adopted a single conformation previously seen in cryogenic X-ray studies, whereas the XFEL data revealed multiple conformations, as seen in previous room-temperature X-ray studies. So, our work reveals that temperature is a defining difference between structures solved using XFELs and those solved using microED. For many proteins, different conformations are visible at cryogenic temperatures when compared to room temperature. This means that these two methods provide complementary views into the molecular landscape, in addition to enabling collection of high-resolution data from small crystals. | Our model system for this work was Cyclophilin A (CypA), which we had previously studied at multiple temperatures. As it turned out, both microED and XFELs enabled us to solve high-resolution structures of CypA, but the conformations of the protein were not the same. In the microED structure, CypA adopted a single conformation previously seen in cryogenic X-ray studies, whereas the XFEL data revealed multiple conformations, as seen in previous room-temperature X-ray studies. So, our work reveals that temperature is a defining difference between structures solved using XFELs and those solved using microED. For many proteins, different conformations are visible at cryogenic temperatures when compared to room temperature. This means that these two methods provide complementary views into the molecular landscape, in addition to enabling collection of high-resolution data from small crystals. | ||
| - | [[Image:Image17.png|left| | + | [[Image:Image17.png|left|400px|thumb]] |
{{Clear}} | {{Clear}} | ||
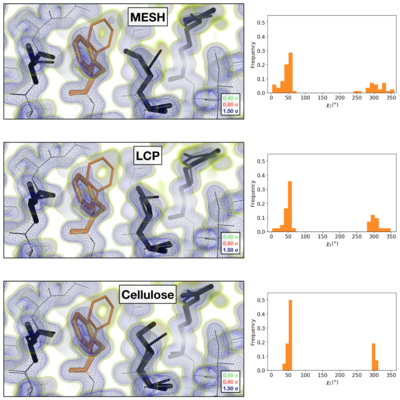
Comparison of the 2mFoFc map and the refined multi-conformer model produced from each serial XFEL experiment. Maps were visualized at multiple contour levels to show evidence for alternative conformations (see static image above). Following multi-conformer refinement, ensembles were generated from each model using phenix.ensemble_refine. In the right panel, a histogram of the Chi1 angles for residue 113 is plotted for the ensemble. Multi-conformer models plus maps, and the distribution of Chi1 angles across the ensemble models are similar for all three XFEL data sets: | Comparison of the 2mFoFc map and the refined multi-conformer model produced from each serial XFEL experiment. Maps were visualized at multiple contour levels to show evidence for alternative conformations (see static image above). Following multi-conformer refinement, ensembles were generated from each model using phenix.ensemble_refine. In the right panel, a histogram of the Chi1 angles for residue 113 is plotted for the ensemble. Multi-conformer models plus maps, and the distribution of Chi1 angles across the ensemble models are similar for all three XFEL data sets: | ||
Revision as of 15:02, 2 February 2020
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.