Superoxide Dismutase
From Proteopedia
(Difference between revisions)
| (27 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='' size='350' side='right' scene='Superoxide_Dismutase/Opening_sod/2' caption='Superoxide dismutase with Cu+2 (orange) and Zn+2 (grey) ions (PDB code [[1spd]])'> | |
| - | + | {{clear}} | |
| + | '''Superoxide dismutase (SOD)''' are a group of antioxidant enzymes which catalyze the dismutation of superoxide to oxygen and hydrogen peroxide. SODs are critical antioxidative proteins, protecting the host from oxidative damage. See also<br /> | ||
| + | * [[Molecular Playground/ Copper-Zinc Superoxide Dismutase]]<br /> | ||
| + | * [[Copper, Zinc Superoxide Dismutase]]<br /> | ||
| + | * [[Human MnSOD and Cancer Research]]<br /> | ||
| + | * [[Molecular Playground/Nickel Superoxide Dismutase]]. | ||
| - | + | For SOD with nitrotyrosine modification see [[Nitrotyrosine]]. | |
<br /> | <br /> | ||
| - | |||
| - | {{TOC limit|limit=2}} | ||
==Types of SOD:== | ==Types of SOD:== | ||
| Line 14: | Line 17: | ||
==Structure:== | ==Structure:== | ||
[[Image:601px-2SOD ribbon pastel.jpg|300px|left]] | [[Image:601px-2SOD ribbon pastel.jpg|300px|left]] | ||
| - | + | ||
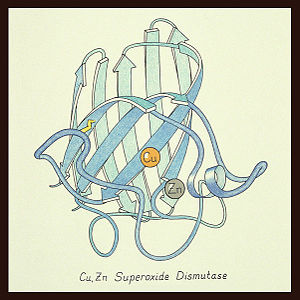
The function of <scene name='Superoxide_Dismutase/Second_opening_sod/3'>SOD</scene> was first determined by Irwin Fridovich and Joe McCord in 1969<ref>PMID:5389100</ref><ref>PMID:2855736</ref>, with the structure of bovine <scene name='Superoxide_Dismutase/Second_opening_sod_cuzn/1'>Cu-Zn</scene> SOD solved soon after by David and Jane Richardson ''et al.'' <ref>PMID:1055410</ref><ref>PMID:7175933</ref> The typical SOD structure is an 8-stranded “Greek Key” beta-barrel with an active site buried between the <scene name='Superoxide_Dismutase/Second_beta_barrel/2'>barrel</scene> and <scene name='Superoxide_Dismutase/Second_opening_sod_loops/1'>two surface loops</scene>. While the typical biological unit for SOD is either dimer or tetramer, monomer forms exist. The Image at the left, drawn by Jane Richardson, illustrates the Greek-Key structure and position of the metal cations. | The function of <scene name='Superoxide_Dismutase/Second_opening_sod/3'>SOD</scene> was first determined by Irwin Fridovich and Joe McCord in 1969<ref>PMID:5389100</ref><ref>PMID:2855736</ref>, with the structure of bovine <scene name='Superoxide_Dismutase/Second_opening_sod_cuzn/1'>Cu-Zn</scene> SOD solved soon after by David and Jane Richardson ''et al.'' <ref>PMID:1055410</ref><ref>PMID:7175933</ref> The typical SOD structure is an 8-stranded “Greek Key” beta-barrel with an active site buried between the <scene name='Superoxide_Dismutase/Second_beta_barrel/2'>barrel</scene> and <scene name='Superoxide_Dismutase/Second_opening_sod_loops/1'>two surface loops</scene>. While the typical biological unit for SOD is either dimer or tetramer, monomer forms exist. The Image at the left, drawn by Jane Richardson, illustrates the Greek-Key structure and position of the metal cations. | ||
==Biochemistry== | ==Biochemistry== | ||
| - | Superoxide is a highly reactive oxygen species and is a major source of oxidative stress in the body, reacting with cellular targets, often causing oxidative damage. <ref>PMID:7493016</ref> SOD protects the body by safely metabolizing the superoxide into unreactive oxygen and hydrogen peroxide. Experiments conducted with knockout mice unable to produce SOD develop widespread oxidative damage and hepatocarcinogenesis and exhibit a greatly reduced lifespan. <ref> PMID:15531919</ref> In humans, mutations to SOD1, one of 3 types found in the human body, can cause familial ALS, a motor neuron disease better known as Lou Gherig’s Disease <ref> PMID:10970056</ref>, and has also been linked to Down’s Syndrome<ref>PMID:7999984</ref>. | + | Superoxide is a highly reactive oxygen species and is a major source of oxidative stress in the body, reacting with cellular targets, often causing oxidative damage. <ref>PMID:7493016</ref> SOD protects the body by safely metabolizing the superoxide into unreactive oxygen and hydrogen peroxide. Experiments conducted with knockout mice unable to produce SOD develop widespread oxidative damage and hepatocarcinogenesis and exhibit a greatly reduced lifespan. <ref> PMID:15531919</ref> In humans, mutations to SOD1, one of 3 types found in the human body, can cause familial ALS, a motor neuron disease better known as Lou Gherig’s Disease <ref> PMID:10970056</ref>, and has also been linked to Down’s Syndrome<ref>PMID:7999984</ref>. |
| + | |||
| + | === Carbon monoxide binding to the heme group at the dimeric interface modulates structure and copper accessibility in the Cu,Zn superoxide dismutase from ''Haemophilus ducreyi'': in silico and in vitro evidences <ref>DOI 10.1080/07391102.2012.680028</ref>=== | ||
| + | <scene name='Journal:JBSD:9/Cv/5'>Superoxide dismutases</scene> (SODs) are metalloenzymes playing a vital role in the defense mechanism against the oxidative stress; they catalyze the dismutation of superoxide, the one-electron reduction product of oxygen, to hydrogen peroxide and molecular oxygen, thus protecting living organism from oxidative lethality (<scene name='Journal:JBSD:9/Cv/7'>conserved SODs regions</scene> among prokaryotes and eukaryotic organisms are highlighted in different colors: <font color='crimson'><b>SS subloop (residues Glu73-Gly93) in crimson</b></font>; (<span style="color:salmon;background-color:black;font-weight:bold;">Zn subloop (residues Gly94-Ala119) in salmon</span>; (<span style="color:violet;background-color:black;font-weight:bold;">Greek key loop (residues Pro135-Gly145) in violet color</span>; (<span style="color:pink;background-color:black;font-weight:bold;">7,8 loop (residues Ala152-Pro169) in pink color)</span>. ''Haemophilus ducreyi'', the causative agent of the sexually transmitted human genital ulcerative disease known as chancroid, expresses one of the most interesting examples of bacterial Cu,Zn SOD (#HdSOD) with the unique feature of binding a heme molecule at the interface between the two subunits asymmetrically bound by residues <scene name='Journal:JBSD:9/Cv/11'>His64 and His124 of subunits A and B</scene>, respectively (<span style="color:lime;background-color:black;font-weight:bold;">His64 and His124 are colored green</span> and <span style="color:yellow;background-color:black;font-weight:bold;">heme molecule is in yellow)</span>. The heme molecule proved to be able to bind small gaseous ligands, such as nitric oxide or carbon monoxide, as a sixth ligand thus displacing the distal histidine. In this study the structural and dynamic response of HdSOD to the <scene name='Journal:JBSD:9/Cv/12'>binding of CO to heme</scene> (<font color='magenta'><b>the carbon atom of CO colored in magenta</b></font> and <font color='red'><b>the oxigen one is in red</b></font>) was studied by means of a combinations of Molecular Dynamics Simulation and X-Ray absorbtion spectroscopy and hypothesis formulated were further confirmed by in vitro experiments. All together the collected results evidenced that binding of the CO molecule produces a strong reduction in the asymmetric fluctuations of the two subunits and long range effects of the heme group on the <scene name='Journal:JBSD:9/Cv/13'>Cu active site structure</scene> (<span style="color:cyan;background-color:black;font-weight:bold;">active site residues: His70, His72, His95, His104, His113, Asp 116 and His 151 are in cyan</span>, <font color='gray'><b>Zn is in gray</b></font> and <span style="color:darkgoldenrod;background-color:black;font-weight:bold;">Cu is in darkgoldenrod</span>) which becomes more easily accessible to the solvent thus causing an increase in copper dismutasic activity. Based on this picture, we suggest a role of HdSOD as a heme-based-sensor protein in which conformational changes, triggered by the heme group, could help ''Haemophilus ducreyi'' to adapt at fluctuating levels of gaseous molecules, such as carbon monoxide or nitric oxide: in the presence of these gasses, the HdSOD would be able to increase its superoxide dismutation activity to subtract superoxide substrate and to prevent the formation of the dangerous peroxynitrite, the molecule generated by the reaction of nitric oxide with superoxide, which is even more harmful to the cell than superoxide. Further investigation to validate this attractive hypothesis is thus desirable. | ||
==Additional Resources== | ==Additional Resources== | ||
See: [[Copper, Zinc Superoxide Dismutase]] <br/> | See: [[Copper, Zinc Superoxide Dismutase]] <br/> | ||
| - | + | == 3D Structures of superoxide dismutase == | |
| - | == 3D Structures of | + | [[Superoxide dismutase 3D structures]] |
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==References== | ==References== | ||
Current revision
| |||||||||||
References
- ↑ McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049-55. PMID:5389100
- ↑ McCord JM, Fridovich I. Superoxide dismutase: the first twenty years (1968-1988). Free Radic Biol Med. 1988;5(5-6):363-9. PMID:2855736
- ↑ Richardson J, Thomas KA, Rubin BH, Richardson DC. Crystal structure of bovine Cu,Zn superoxide dismutase at 3 A resolution: chain tracing and metal ligands. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1349-53. PMID:1055410
- ↑ Tainer JA, Getzoff ED, Beem KM, Richardson JS, Richardson DC. Determination and analysis of the 2 A-structure of copper, zinc superoxide dismutase. J Mol Biol. 1982 Sep 15;160(2):181-217. PMID:7175933
- ↑ Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995 Dec;11(4):376-81. PMID:7493016 doi:http://dx.doi.org/10.1038/ng1295-376
- ↑ Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005 Jan 13;24(3):367-80. PMID:15531919
- ↑ Al-Chalabi A, Leigh PN. Recent advances in amyotrophic lateral sclerosis. Curr Opin Neurol. 2000 Aug;13(4):397-405. PMID:10970056
- ↑ Groner Y, Elroy-Stein O, Avraham KB, Schickler M, Knobler H, Minc-Golomb D, Bar-Peled O, Yarom R, Rotshenker S. Cell damage by excess CuZnSOD and Down's syndrome. Biomed Pharmacother. 1994;48(5-6):231-40. PMID:7999984
- ↑ Chillemi G, De Santis S, Falconi M, Mancini G, Migliorati V, Battistoni A, Pacello F, Desideri A, D'Angelo P. Carbon monoxide binding to the heme group at the dimeric interface modulates structure and copper accessibility in the Cu,Zn superoxide dismutase from Haemophilus ducreyi: in silico and in vitro evidences. J Biomol Struct Dyn. 2012 Jul;30(3):269-79. Epub 2012 Jun 11. PMID:22686457 doi:10.1080/07391102.2012.680028
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Alexander Berchansky, Eric Martz, Joel L. Sussman, Jane S. Richardson, Jaime Prilusky