User:Samantha Schneider/Sandbox1
From Proteopedia
< User:Samantha Schneider(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 15: | Line 15: | ||
== Function == | == Function == | ||
| - | Factor XIII is a [[tranglutaminase]] that circulates throughout the blood as a heterotetramer. The B subunits bind to the clot structure. When fibrin is present, thrombin cleaves the <scene name='84/842930/Sessile_bond/1'>bond</scene> between the A and B subunits and the R37-G38 peptide bond in the A subunit that reveals it's active enzymatic region at the N-terminus. Calcium ions further activate the A subunits through a change in shape. The calcium ions additionally dissociate the non-covalently bound B subunits. The remaining dimer of two active A subunits, FactorXIIIa, crosslinks fibrin by forming isopeptide bonds between glutamines and lysines within the fibrin< | + | Factor XIII is a [[tranglutaminase]] that circulates throughout the blood as a heterotetramer. The B subunits bind to the clot structure. When fibrin is present, thrombin cleaves the <scene name='84/842930/Sessile_bond/1'>bond</scene> between the A and B subunits and the R37-G38 peptide bond in the A subunit that reveals it's active enzymatic region at the N-terminus. Calcium ions further activate the A subunits through a change in shape. The calcium ions additionally dissociate the non-covalently bound B subunits. The remaining dimer of two active A subunits, FactorXIIIa, crosslinks fibrin by forming isopeptide bonds between glutamines and lysines within the fibrin<sup>2</sup> The crosslinks make the clot more durable and more resistant to fibrinolysis due to premature enzymatic degradation. It additionally has been found to play a role in proper wound healing, carrying pregnancy to full term, and in the development of new blood vessels. |
| - | FXIIIa catalyzes the formation of Nε(y-glutamyl)lysine protein to protein side chain bridges within the clot network< | + | FXIIIa catalyzes the formation of Nε(y-glutamyl)lysine protein to protein side chain bridges within the clot network <sup>2</sup>. Fibrin fibers become thinner and longer once stabilized by FXIIIa although this doe not affect the clot density in any way. The cross-linking dramatically increases the clot stability and resistance to degradation. There is a transamidation reaction between Gln and Lys residues of neighboring molecules<sup>1</sup>. |
| Line 29: | Line 29: | ||
In Class I cases, which constitute the majority of FXIII deficiency cases, there is virtually no thrombin dependent transamidase activity. In class 2 cases there has been a Val34Leu mutation in the A subunits. The class II mutation leads to a two-fold increase in FXIII. This mutation has shown to have protective effects against thrombotic disease in its population. | In Class I cases, which constitute the majority of FXIII deficiency cases, there is virtually no thrombin dependent transamidase activity. In class 2 cases there has been a Val34Leu mutation in the A subunits. The class II mutation leads to a two-fold increase in FXIII. This mutation has shown to have protective effects against thrombotic disease in its population. | ||
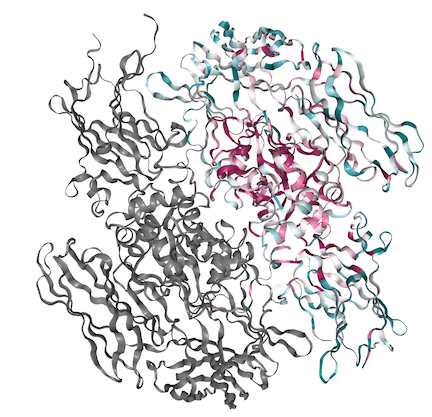
| - | == | + | == Sequence Conservation == |
[[Image:FXIIIstructure_conserved.png]] | [[Image:FXIIIstructure_conserved.png]] | ||
Current revision
Human Coagulation Factor XIII
| |||||||||||
References
- ↑ Gupta, S. et al. Revisiting the mechanism of coagulation factor XIII activation and regulation from a structure/functional perspective. Sci. Rep. 6, 30105; doi: 10.1038/srep30105 (2016)
- ↑ Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.
- ↑ https://rarediseases.org/rare-diseases/factor-xiii-deficiency/