We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Daniel Seeman
From Proteopedia
(Difference between revisions)
m |
m |
||
| (20 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <center>[ | + | <center><span class="plainlinks">'''[https://www.linkedin.com/in/daniel-seeman Daniel P. Seeman, PhD (Senior Scientist)]''' |
| - | '' | + | |
| - | + | ||
| + | |||
| + | [[Image:delphiproteins.png|center|thumb|400px|Electrostatic potentials of three proteins (β-lactoglobulin, Bovine serum albumin, and Zn-Insulin) at pH 6. Calculated with DelPhi (a 'Nonlinear Poisson Boltzmann Solver') and displayed using UCSF Chimera. Protein charge anisotropy is a major component of both protein self-association, ''and'' interactions with bio-derived polyelectrolytes.]] | ||
| + | |||
| + | |||
| + | |||
| + | |||
=== About proteopedia: === | === About proteopedia: === | ||
| Line 8: | Line 14: | ||
:'''<span style="color:cyan">PDB seed</span>''': automatically generated page for pdb files. | :'''<span style="color:cyan">PDB seed</span>''': automatically generated page for pdb files. | ||
:'''User pages''': ''this'' page, and others like it | :'''User pages''': ''this'' page, and others like it | ||
| - | |||
| - | === new page(s): === | ||
| - | *[[User:Daniel Seeman/Alpha-1-antitrypsin]] | ||
| - | *[[User:Daniel Seeman/Caspase-7 Dynamics]] | ||
| - | *[[Alpha-1-antitrypsin]] | ||
| - | *[[Molecular_Playground/BLG]] | ||
Current revision
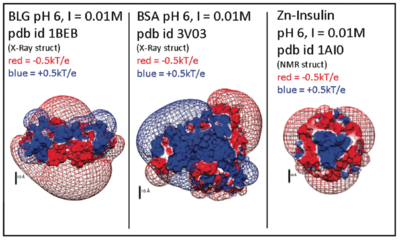
Electrostatic potentials of three proteins (β-lactoglobulin, Bovine serum albumin, and Zn-Insulin) at pH 6. Calculated with DelPhi (a 'Nonlinear Poisson Boltzmann Solver') and displayed using UCSF Chimera. Protein charge anisotropy is a major component of both protein self-association, and interactions with bio-derived polyelectrolytes.
About proteopedia:
- Topic Pages
