User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
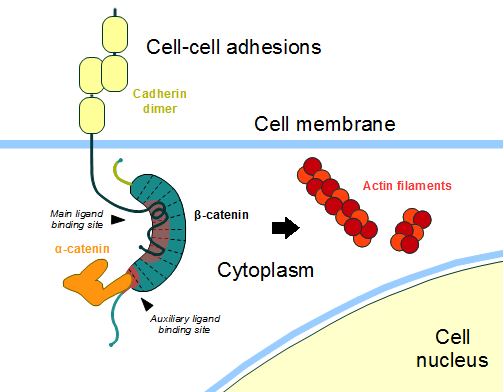
| - | ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota ([https://en.wikipedia.org/wiki/Eukaryote Eukaryota]) phylum, in humans the gene [ | + | ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota ([https://en.wikipedia.org/wiki/Eukaryote Eukaryota]) phylum, in humans the gene CTNNB1 ([https://www.ncbi.nlm.nih.gov/gene/1499 CTNNB1]) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway <ref name=logan&nusse2004> DOI : 10.1146/annurev.cellbio.20.010403.113126</ref> (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. <ref name="xing2009">DOI 10.1016/j.str.2007.12.021</ref> |
<StructureSection load='2Z6G' size='400' caption='Structure of ß-catenin from Zebrafish' scene='84/848919/Dotsbetacateninacoloridaartigo/1'> | <StructureSection load='2Z6G' size='400' caption='Structure of ß-catenin from Zebrafish' scene='84/848919/Dotsbetacateninacoloridaartigo/1'> | ||
| Line 13: | Line 13: | ||
In contrast to the armadillo ligand-binding structural groove, the C-terminal tail is highly negatively charged. The C-helix caps the {{Template:ColorKey_Hydrophobic}} <scene name='84/848919/Hydrophilichelixc/1'>surface formed by the C-terminal end of the last armadillo repeats.</scene>. However, the other side of the surface, exposed to solvent, is composed of {{Template:ColorKey_Polar}} residues. Thereby, this structure forms part of the superhelical structure core of ß-catenin together with armadillo repeat domain. <ref name="xing2009" /> | In contrast to the armadillo ligand-binding structural groove, the C-terminal tail is highly negatively charged. The C-helix caps the {{Template:ColorKey_Hydrophobic}} <scene name='84/848919/Hydrophilichelixc/1'>surface formed by the C-terminal end of the last armadillo repeats.</scene>. However, the other side of the surface, exposed to solvent, is composed of {{Template:ColorKey_Polar}} residues. Thereby, this structure forms part of the superhelical structure core of ß-catenin together with armadillo repeat domain. <ref name="xing2009" /> | ||
| - | It is possible that | + | It is possible that the C-helix is important for the transactivation of Wnt-responsive genes, but not for the cell adhesion through [[Cadherin|cadherins]]. Hence, this same β-catenin region is also the binding site of transcriptional inhibitors that compete directly with TCF for β-catenin binding.<ref name="xing2009" /> |
==Cell Adhesion== | ==Cell Adhesion== | ||
| Line 25: | Line 25: | ||
==The ß-catenin destruction complex== | ==The ß-catenin destruction complex== | ||
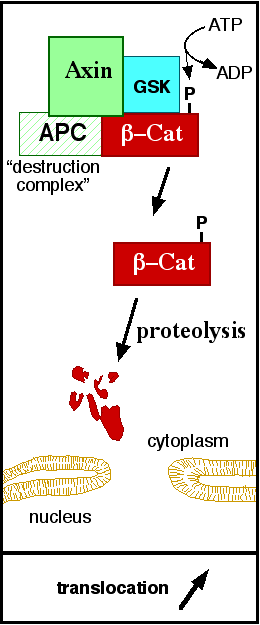
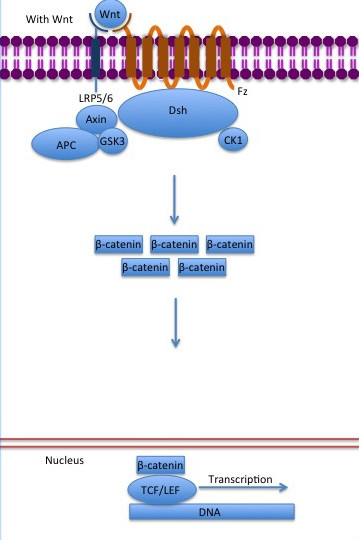
| - | In baseline conditions without Wnt signaling, ß-catenin concentrations are low in both the cytoplasm and the nucleus. Then, the destruction complex (Figure 2), formed by APC, [[Axin]], CK1ɑ and [[Glycogen synthase kinase 3|GSK]], is active and causes the degradation of the protein through proteasome. Initially it is recognized by APC and [[Axin]] that promote the phosphorylation of Ser45 by CK1ɑ. This facilitates the phosphorylation by [[Cyclin-dependent kinase|GSK]] in the residues of the amino acids Thr41, Ser37 and Ser33. The last two, when phosphorylated, leads to recognition by ß-TrCP and consequently ubiquitination by an [[Ubiquitin protein ligase|E3 ligase]] and degradation by [[Proteasome|26S proteasome]]. <ref name="valenta2012" /> Furthermore, the relation Wnt/ß-catenin pathway usually are reported by 'canonical' and 'non-canonical', whose meaning remotes to the components of the cascate. The first leads to accumulation and stabilization of cytosolic (unphosphorylated) ß-catenin and the second promotes the increase in intracellular calcium or mediate cell polarity, but both are established in embryonic development of normal tissue and organs. <ref name=Arend ''et al''2013> | + | In baseline conditions without Wnt signaling, ß-catenin concentrations are low in both the cytoplasm and the nucleus. Then, the destruction complex (Figure 2), formed by APC, [[Axin]], CK1ɑ and [[Glycogen synthase kinase 3|GSK]], is active and causes the degradation of the protein through proteasome. Initially it is recognized by APC and [[Axin]] that promote the phosphorylation of Ser45 by CK1ɑ. This facilitates the phosphorylation by [[Cyclin-dependent kinase|GSK]] in the residues of the amino acids Thr41, Ser37 and Ser33. The last two, when phosphorylated, leads to recognition by ß-TrCP and consequently ubiquitination by an [[Ubiquitin protein ligase|E3 ligase]] and degradation by [[Proteasome|26S proteasome]]. <ref name="valenta2012" /> Furthermore, the relation Wnt/ß-catenin pathway usually are reported by 'canonical' and 'non-canonical', whose meaning remotes to the components of the cascate. The first leads to accumulation and stabilization of cytosolic (unphosphorylated) ß-catenin and the second promotes the increase in intracellular calcium or mediate cell polarity, but both are established in embryonic development of normal tissue and organs. <ref name=Arend ''et al''2013></ref>Arend ''et al,''2013.The Wnt/β-catenin pathway in ovarian cancer: A review. Gynecologic Oncology. Volume 131, Issue 3, December 2013, Pages 772-779.</ref> <ref name=Takayama ''et al''1996>Takayama ''et al,'' 1996. ß-Catenin Expression in Human Cancers. American journal of Pathology, Vol. 148, No. 1, January. </ref> |
[[Image:Axindestructioncomplex.png]] | [[Image:Axindestructioncomplex.png]] | ||
Revision as of 02:36, 22 June 2020
ß-catenin
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||