User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
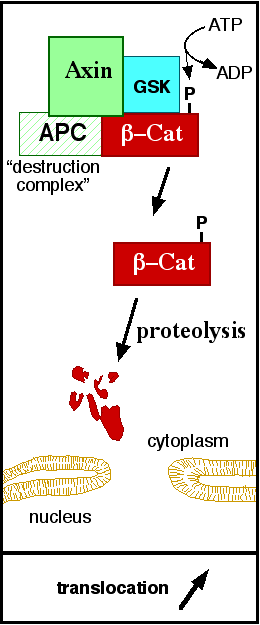
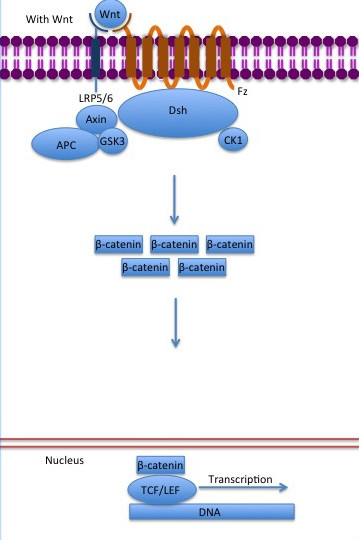
The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure 3) leads to increased levels of the protein in cytoplasm and its translocation into the nucleus. ß-catenin interacts with different nuclear pore complex components and ARM repeats <scene name='84/848919/R10-12/1'>R10-R12</scene> are critical for its import and export. [[Forkhead box protein|FoxM1]] also facilitates its nuclear translocation directly interacting with ARM repeats <scene name='84/848919/R11-12/2'>R11-R12</scene>. [[Forkhead box protein|FoxM1]] forms a complex with ß-catenin/TCF on the promoters of Wnt target genes. Once in the nucleus, ß-catenin and its DNA binding partners can activate transcription of Wnt/ß-catenin target genes. Therefore, ß-catenin can only initiates transcription in a multimeric complex, as its central transcriptional activator. <ref name="valenta2012" /> | The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure 3) leads to increased levels of the protein in cytoplasm and its translocation into the nucleus. ß-catenin interacts with different nuclear pore complex components and ARM repeats <scene name='84/848919/R10-12/1'>R10-R12</scene> are critical for its import and export. [[Forkhead box protein|FoxM1]] also facilitates its nuclear translocation directly interacting with ARM repeats <scene name='84/848919/R11-12/2'>R11-R12</scene>. [[Forkhead box protein|FoxM1]] forms a complex with ß-catenin/TCF on the promoters of Wnt target genes. Once in the nucleus, ß-catenin and its DNA binding partners can activate transcription of Wnt/ß-catenin target genes. Therefore, ß-catenin can only initiates transcription in a multimeric complex, as its central transcriptional activator. <ref name="valenta2012" /> | ||
| - | TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT (orange), which interacts with the central ARM repeat of ß-catenin (green).</scene> ([http://www.rcsb.org/structure/1M1E 1M1E | + | TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT (here shown in orange, full length ICAT from ''Homo sapiens''), which interacts with the central ARM repeat of ß-catenin (shown in green, from ''Mus musculus'').</scene> ([http://www.rcsb.org/structure/1M1E 1M1E]) <ref name="valenta2012" /> |
| + | This interaction can be divided in two regions: the ICAT extended C-terminal region bind to the ß-catenin ARM 5-10 and the <scene name='84/848919/Helicalicatdomain_bcat11_12/1'>ICAT helical N-terminal domain interacts with the ARM repeat 11 and 12</scene>. The first one overlaps with others ß-catenin ligands and is known for its several hydrophobic interactions and two salt bridges - Asp66 and Glu75 form salt bridges with ß-catenin residues Lys435 (repeat 8) and Lys312 (repeat 5) respectively, and Val68, Met69, and Phe71 interact with hydrophobic pockets on the surface of ß-catenin. The conserved acids are further stabilized by other polar contacts: ICAT Asp66 binds to ß-catenin Arg474 (repeat 9), and ICAT Glu75 binds to the backbone amide of ß-catenin Gly307 (repeat 5). Finally, the interaction between the ICAT helical domain and the two last ARM repeat is mediated by water contact (with ARM 11) and hydrophobic interactions (ARM 12). The hydrophobic interaction is stabilized by the connections between the aliphatic portion of the Lys19 side chain and the aromatic rings of Phe660 (ARM 12) and Phe40 (ICAT). . Lys19 also forms a salt bridge with -catenin Glu664 (repeat 12). Another polar interaction occurs between ICAT Glu37 and -catenin Arg661. | ||
[[Image:Canonical Wnt pathway with Wnt..jpg]] | [[Image:Canonical Wnt pathway with Wnt..jpg]] | ||
Revision as of 14:37, 9 July 2020
ß-catenin
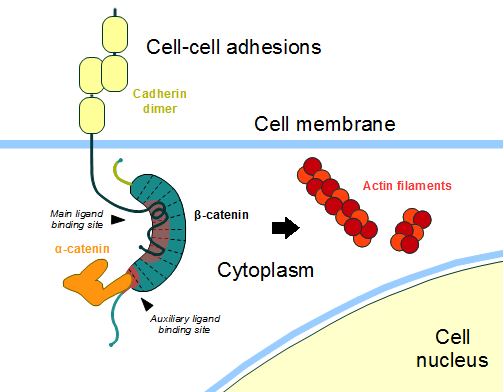
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||