User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 35: | Line 35: | ||
TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT, which interacts with the central ARM repeat of ß-catenin</scene> (here ICAT is represented in orange and is a full length structure from ''Homo sapiens''; ß-catenin is shown in green and is from ''Mus musculus''). ([http://www.rcsb.org/structure/1M1E 1M1E]) <ref name="valenta2012" /> | TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT, which interacts with the central ARM repeat of ß-catenin</scene> (here ICAT is represented in orange and is a full length structure from ''Homo sapiens''; ß-catenin is shown in green and is from ''Mus musculus''). ([http://www.rcsb.org/structure/1M1E 1M1E]) <ref name="valenta2012" /> | ||
| - | This interaction can be divided in two regions: the <scene name='84/848919/Extendedregionicat_bcat_5_10/1'>ICAT extended C-terminal region bind to the ß-catenin ARM 5-10</scene> and the <scene name='84/848919/Helicalicatdomain_bcat11_12/2'>ICAT helical N-terminal domain interacts with the ARM repeat 11 and 12</scene>. The first one overlaps with others ß-catenin ligands and is known for its several hydrophobic interactions and <scene name='84/848919/2saltbridge_icat_bcat/1'> two salt bridges </scene> - Asp66 and Glu75 form salt bridges with ß-catenin residues Lys435 (repeat 8) and Lys312 (repeat 5). | + | This interaction can be divided in two regions: the <scene name='84/848919/Extendedregionicat_bcat_5_10/1'>ICAT extended C-terminal region bind to the ß-catenin ARM 5-10</scene> and the <scene name='84/848919/Helicalicatdomain_bcat11_12/2'>ICAT helical N-terminal domain interacts with the ARM repeat 11 and 12</scene>. The first one overlaps with others ß-catenin ligands and is known for its several <scene name='84/848919/Hydrophobic_icat_bcat/1'>hydrophobic interactions</scene> (for example, Val68, Met69, and Phe71 interact with hydrophobic sites on the surface of ß-catenin) and <scene name='84/848919/2saltbridge_icat_bcat/1'> two salt bridges </scene> - Asp66 and Glu75 form salt bridges with ß-catenin residues Lys435 (repeat 8) and Lys312 (repeat 5). There are other polar contacts to stabilize the protein-protein binding. Finally, the interaction between the ICAT helical domain and the two last ARM repeat is water-mediated contact (with ARM 11) and hydrophobic interactions (ARM 12). The hydrophobic interactions are stabilized by the connections between the aliphatic portion of the <scene name='84/848919/Hydrophbic_lys19_icat_bcat/1'>Lys19 side chain and the aromatic rings of Phe660 (ARM 12) and Phe40 (ICAT)</scene>. Lys19 also forms a <scene name='84/848919/Salt_bridge_lys19_icat_bcat/1'>salt bridge</scene> with ß-catenin Glu664 (repeat 12). Another polar interaction occurs between <scene name='84/848919/Arg_glu_icat_bcat/1'>ICAT Glu37 and ß-catenin Arg661</scene>. |
[[Image:Canonical Wnt pathway with Wnt..jpg]] | [[Image:Canonical Wnt pathway with Wnt..jpg]] | ||
Revision as of 17:12, 9 July 2020
ß-catenin
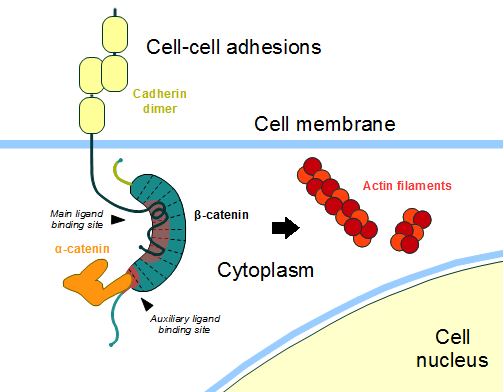
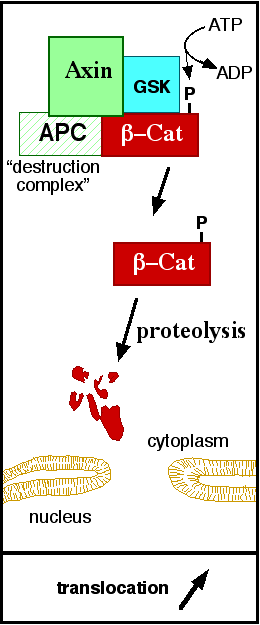
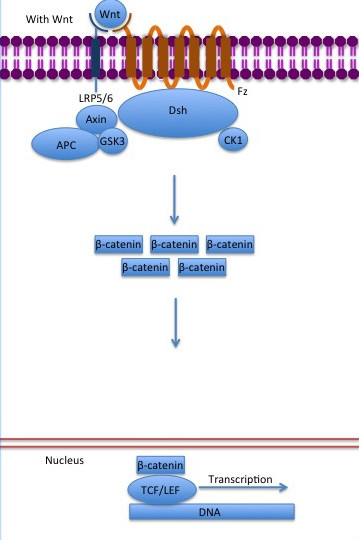
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||