User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
< User:Isabela Fonseca de Oliveira Granha(Difference between revisions)
| (17 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | = | + | =Crystal Structure of a Full-Length Zebrafish Beta-Catenin= |
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota ([https://en.wikipedia.org/wiki/Eukaryote Eukaryota]) phylum, in humans the gene CTNNB1 ([https://www.ncbi.nlm.nih.gov/gene/1499 CTNNB1]) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway <ref name=logan&nusse2004> DOI : 10.1146/annurev.cellbio.20.010403.113126</ref> (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. <ref name="xing2009">DOI 10.1016/j.str.2007.12.021</ref> | ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota ([https://en.wikipedia.org/wiki/Eukaryote Eukaryota]) phylum, in humans the gene CTNNB1 ([https://www.ncbi.nlm.nih.gov/gene/1499 CTNNB1]) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway <ref name=logan&nusse2004> DOI : 10.1146/annurev.cellbio.20.010403.113126</ref> (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. <ref name="xing2009">DOI 10.1016/j.str.2007.12.021</ref> | ||
| Line 6: | Line 6: | ||
==Structure== | ==Structure== | ||
| - | The zebrafish ([https://pt.wikipedia.org/wiki/Danio_rerio ''Danio rerio'']) <scene name='84/848919/Betacateninacoloridaartigo/2'>ß-catenin</scene> ([http://www.rcsb.org/structure/2Z6G 2Z6G]) contains residues 126-681 and a central core of <scene name='84/848919/Armrepeatsdomain/1'>12 armadillo repeats domain</scene> and an alpha helix, the <scene name='84/848919/C-helix3correta/1'>helix-C</scene>, at the | + | The zebrafish ([https://pt.wikipedia.org/wiki/Danio_rerio ''Danio rerio'']) <scene name='84/848919/Betacateninacoloridaartigo/2'>ß-catenin</scene> ([http://www.rcsb.org/structure/2Z6G 2Z6G]) contains residues 126-681 and a central core of <scene name='84/848919/Armrepeatsdomain/1'>12 armadillo repeats domain</scene> and an alpha helix, the <scene name='84/848919/C-helix3correta/1'>helix-C</scene>, at the ß-catenin C-terminal domain. |
| - | The terminal domains sequences | + | The terminal domains sequences mediate some of the protein interactions and are negatively charged (Figure 1). It is observed that the helix-C constitutes the C-terminal domain. The N terminus of the first armadillo repeat has an <scene name='84/848919/Correton-terminushelix/1'>extra alpha helix</scene>. Both N- and C-terminal domains do not interact specifically with the armadillo repeat domain. <ref name="xing2009" /> |
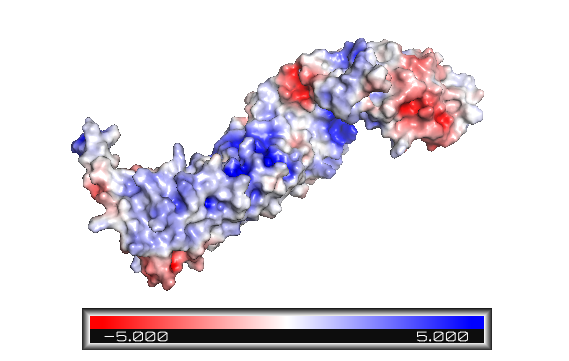
| - | In contrast to the armadillo ligand-binding structural groove, the C-terminal tail is highly negatively charged. The C-helix caps the {{Template:ColorKey_Hydrophobic}} <scene name='84/848919/ | + | The armadillo domain is more conserved than the terminal domains. It is made of 12 armadillo repeats each one with <scene name='84/848919/Centerarm5_helices/1'>three alpha helices connected by loops</scene> (as shown in ARM repeat 5), except for the <scene name='84/848919/Centerarm_repeat_7/1'>ARM repeat 7, which has two helices</scene>. Furthermore, it has a particular site which is positively charged (Figure 1), constituting the binding surface for the majority of ß-catenin ligands. Because the armadillo domain is positively while the terminal tails are negatively charged (Figure 1), their interactions are nonspecific. It is proposed that both tails act like chaperones - they might avoid nonspecific protein interactions of the ARM repeat domain and its self-aggregation.<ref name="xing2009" /> |
| - | It is possible that the C-helix is important for the transactivation of Wnt-responsive genes, but not for the cell adhesion through [[Cadherin|cadherins]]. Hence, this same β-catenin region is also the binding site of transcriptional inhibitors that compete directly with TCF for β-catenin binding.<ref name="xing2009" /> | + | |
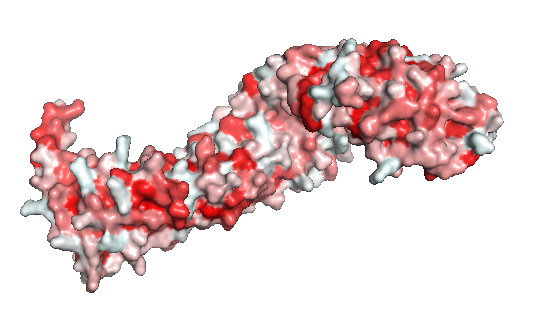
| + | In contrast to the armadillo ligand-binding structural groove, the C-terminal tail is highly negatively charged (Figure 1). The C-helix caps the {{Template:ColorKey_Hydrophobic}} <scene name='84/848919/Centerhydrophilichelixc/1'>surface formed by the C-terminal end of the last armadillo repeats</scene>. However, the other side of the surface, exposed to solvent, is composed of {{Template:ColorKey_Polar}} residues (Figure 2). Thereby, this structure forms part of the superhelical structure core of ß-catenin together with armadillo repeat domain. It is possible that the C-helix is important for the transactivation of Wnt-responsive genes, but not for the cell adhesion through [[Cadherin|cadherins]]. Hence, this same β-catenin region is also the binding site of transcriptional inhibitors that compete directly with TCF for β-catenin binding.<ref name="xing2009" /> | ||
| + | |||
| + | [[Image: Betacatenin eletrostatics white.png]] | ||
| + | '''Figure 1''': Beta-catenin eletrostatic surface. The color blue indicates positively charged sites, and red, negative. The armadillo repeat domain has various positive sites and a particular one is an important binding area. The terminal tails are predominantly represented in red - and the C-helix is highly negatively charged. | ||
| + | |||
| + | [[Image: Whitebetacatenincartoonhidrophocity.png]] | ||
| + | '''Figure 2''': The beta-catenin polarity surface. The red color represents hidrophobic sites, and white, the hidrophilic areas. The protein does not have a partiular polar or apolar area. Polarity is well distributed through the molecule as well as the protein ligands. | ||
| + | |||
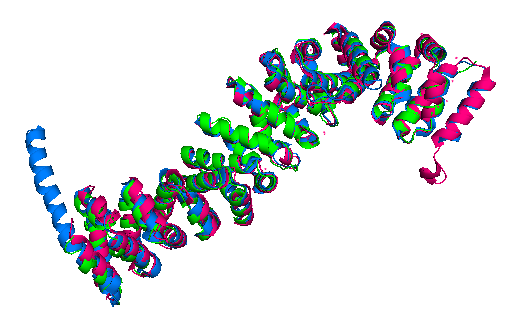
| + | Finally, the ''Danio rerio'' ([http://www.rcsb.org/structure/2Z6G 2Z6G] full length structure, blue), ''Mus musculus'' ([https://www.rcsb.org/structure/2BCT 2BCT] armadillo repeat region, green) and ''Homo sapiens'' ([https://www.rcsb.org/structure/2Z6H 2Z6H] full length structure, pink) beta-catenin alignment (Figure 3) shows that the protein structure is quite similar in these organisms. The three structures have 12 armadillo repeat group and the superposition indicates that the helix C in zebrafish and human beta-catenin conformation and orientation are essentially the same in both crystal structures. This great similarity between these proteins demonstrates that beta-catenin is evolutionary conserved and so are the pathways that it takes part. | ||
| + | |||
| + | [[Image:II2z6g 2bct 2z6h white.png]] | ||
| + | '''Figure 3''': Superposition of a full length zebrafish (shown in blue), full length human (pink) and armadillo repeat region mouse (green) beta-catenin. | ||
==Cell Adhesion== | ==Cell Adhesion== | ||
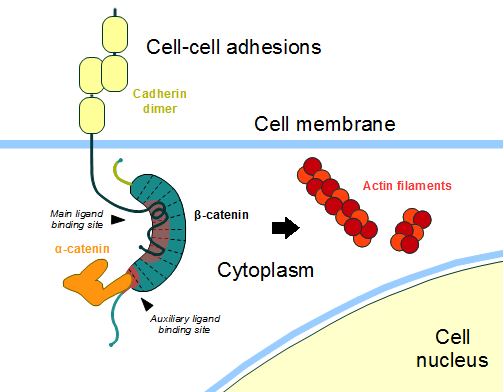
| - | In the absence of Wnt stimulus, ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure | + | In the absence of Wnt stimulus, ß-catenin is located at the cytoplasmic side of the membrane as a component of cadherin-based cell-cell connections (Figure 4). [[Cadherin|Cadherins]] are transmembrane glycoproteins calcium-dependent that mediate cell-cell adhesion through link specially to ß-catenin by their cytoplasmic tails. The cadherin-catenin complex forms adherens junctions that polarize epithelial tissues and hold the cells together. However, in case of some tumor metastasis, that complex is reported as disrupted: in order to become more migratory, epithelial cells must loose their characteristic polarity, thus the complex might be affected (phenomenon described as 'cadherin switching' in epithelial-to-mesenchymal transition, EMT).<ref>Developmental Biology . Eleventh Edition. By Scott F. Gilbert and Michael J. F. Barresi. Sunderland (Massachusetts): Sinauer Associates. ISBN: 978-1-60535-470-5. 2016. </ref> |
| - | The most known interaction occurs between <scene name='84/848919/Beta-catenin_e-cadherin/3'> ß-catenin and E-cadherin</scene>, epithelial cadherin (the ß-catenin residues 134–671 are represented in green and the residues 577–728 of the mature E-cadherin sequence are colored in rose. The proteins are from ''Mus musculus'') (1I7X). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the cadherin. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/2'>interaction surface is extensive</scene>, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> | + | The most known interaction occurs between <scene name='84/848919/Beta-catenin_e-cadherin/3'> ß-catenin and E-cadherin</scene>, epithelial cadherin (the ß-catenin residues 134–671 are represented in green and the residues 577–728 of the mature E-cadherin sequence are colored in rose. The proteins are from ''Mus musculus'') ([https://www.rcsb.org/structure/1i7x 1I7X]). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the cadherin. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/2'>interaction surface is extensive</scene>, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> |
[[Image:Beta-catenin-moonlighting.png]] | [[Image:Beta-catenin-moonlighting.png]] | ||
| - | '''Figure | + | '''Figure 4''': Cadherin-based cell adhesion. Alpha-catenin/ß-catenin forms a heterodimer that can connects to E-cadherin promoting the adherens junctions. As a homodimer, alpha-catenin interacts with actin. Adapted from: Bubus12/CC BY [https://commons.wikimedia.org/wiki/File:Beta-catenin-moonlighting.png] |
==The ß-catenin destruction complex== | ==The ß-catenin destruction complex== | ||
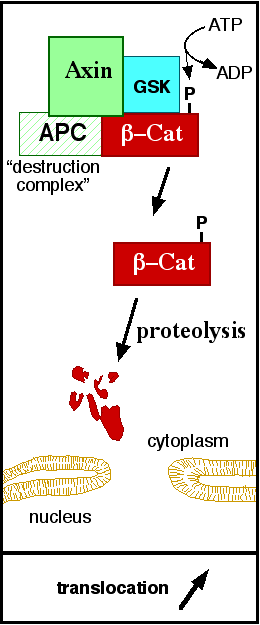
| - | In baseline conditions without Wnt signaling, ß-catenin concentrations are low in both the cytoplasm and the nucleus. Then, the destruction complex (Figure | + | In baseline conditions without Wnt signaling, ß-catenin concentrations are low in both the cytoplasm and the nucleus. Then, the destruction complex (Figure 5), formed by APC, [[Axin]], CK1ɑ and [[Glycogen synthase kinase 3|GSK]], is active and causes the degradation of the protein through proteasome. Initially it is recognized by APC and [[Axin]] that promote the phosphorylation of Ser45 by CK1ɑ. This facilitates the phosphorylation by [[Cyclin-dependent kinase|GSK]] in the residues of the amino acids Thr41, Ser37 and Ser33. The last two, when phosphorylated, leads to recognition by ß-TrCP and consequently ubiquitination by an [[Ubiquitin protein ligase|E3 ligase]] and degradation by [[Proteasome|26S proteasome]]. <ref name="valenta2012" /> Furthermore, the relation Wnt/ß-catenin pathway usually are reported by 'canonical' and 'non-canonical', whose meaning remotes to the components of the cascate. The first leads to accumulation and stabilization of cytosolic (unphosphorylated) ß-catenin and the second promotes the increase in intracellular calcium or mediate cell polarity, but both are established in embryonic development of normal tissue and organs. <ref name=Arend ''et al''2013> Arend ''et al,''2013. The Wnt/β-catenin pathway in ovarian cancer: A review. Gynecologic Oncology. Volume 131, Issue 3, December 2013, Pages 772-779.</ref> <ref name=Takayama ''et al''1996>Takayama ''et al,'' 1996. ß-Catenin Expression in Human Cancers. American journal of Pathology, Vol. 148, No. 1, January. </ref> |
[[Image:Axindestructioncomplex.png]] | [[Image:Axindestructioncomplex.png]] | ||
| - | '''Figure | + | '''Figure 5''': A simplified diagram of the ß-catenin destruction complex. The destruction complex proteins promote the ß-catenin proteolysis in cytoplasm. Source: JWSchmidt at the English language Wikipedia/CC BY-SA [https://commons.wikimedia.org/wiki/File:Axindestructioncomplex.png] |
==DNA binding and transcription== | ==DNA binding and transcription== | ||
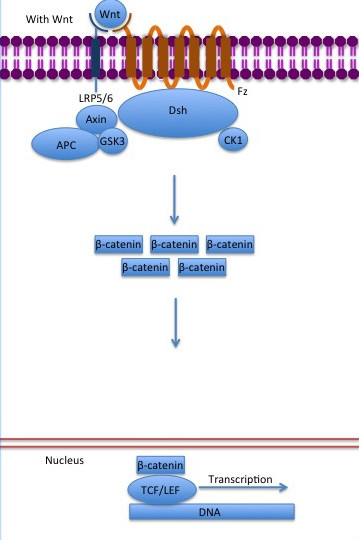
| - | The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure | + | The inhibition of ß-catenin destruction complex through activation of the Wnt pathway (Figure 6) leads to increased levels of the protein in cytoplasm and its translocation into the nucleus. ß-catenin interacts with different nuclear pore complex components and ARM repeats <scene name='84/848919/R10-12/1'>R10-R12</scene> are critical for its import and export. [[Forkhead box protein|FoxM1]] also facilitates its nuclear translocation directly interacting with ARM repeats <scene name='84/848919/R11-12/2'>R11-R12</scene>. [[Forkhead box protein|FoxM1]] forms a complex with ß-catenin/TCF on the promoters of Wnt target genes. Once in the nucleus, ß-catenin and its DNA binding partners can activate transcription of Wnt/ß-catenin target genes. Therefore, ß-catenin can only initiates transcription in a multimeric complex, as its central transcriptional activator. <ref name="valenta2012" /> |
TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT</scene> (here ICAT is represented in orange and is a full length structure from ''Homo sapiens''; ß-catenin is shown in green and is from ''Mus musculus''). ([http://www.rcsb.org/structure/1M1E 1M1E]) <ref name="valenta2012" /> | TCF transcription factors works as the principal nuclear member of ß-catenin multimeric complex. TCFs bind to DNA enhancers and ß-catenin acts as a link in a chain between them and others transcriptional coactivators. This interaction can be modulated to enhance, repress os switch off ß-catenin-mediated transcription. The majority of these transcription coactivators binds to <scene name='84/848919/R12andhelix-c/1'>the last ARM repeat and interacts with Helix-C</scene> and many of them can affect chromatin structure. Indeed, it seems that the C-terminus region of ß-catenin coordinates the recruitment and sequential exchange of these proteins. Binding of ß-catenin to TCF is blocked by some proteins such as <scene name='84/848919/Icat_bcat/3'>ICAT</scene> (here ICAT is represented in orange and is a full length structure from ''Homo sapiens''; ß-catenin is shown in green and is from ''Mus musculus''). ([http://www.rcsb.org/structure/1M1E 1M1E]) <ref name="valenta2012" /> | ||
| - | This interaction can be divided in two regions: the <scene name='84/848919/Extendedregionicat_bcat_5_10/2'>ICAT extended C-terminal region bind to the ß-catenin ARM 5-10 </scene> and the <scene name='84/848919/Helicalicatdomain_bcat11_12/4'>ICAT helical N-terminal domain interacts with the ARM repeat 11 and 12</scene>. The first one overlaps with others ß-catenin ligands and is known for its several <scene name='84/848919/Hydrophobic_icat_bcat/1'>hydrophobic interactions</scene> (for example, Val68, Met69, and Phe71 interact with hydrophobic sites on the surface of ß-catenin) and <scene name='84/848919/2saltbridge_icat_bcat/ | + | This interaction can be divided in two regions: the <scene name='84/848919/Extendedregionicat_bcat_5_10/2'>ICAT extended C-terminal region bind to the ß-catenin ARM 5-10 </scene> and the <scene name='84/848919/Helicalicatdomain_bcat11_12/4'>ICAT helical N-terminal domain interacts with the ARM repeat 11 and 12</scene>. The first one overlaps with others ß-catenin ligands and is known for its several <scene name='84/848919/Hydrophobic_icat_bcat/1'>hydrophobic interactions</scene> (for example, Val68, Met69, and Phe71 interact with hydrophobic sites on the surface of ß-catenin) and <scene name='84/848919/2saltbridge_icat_bcat/2'>two salt bridges</scene> - Asp66 and Glu75 form salt bridges with ß-catenin residues Lys435 (repeat 8) and Lys312 (repeat 5). There are other polar contacts to stabilize the protein-protein binding. Finally, the interaction between the ICAT helical domain and the two last ARM repeat is water-mediated contact (with ARM 11) and hydrophobic interactions (ARM 12). The hydrophobic interactions are stabilized by the connections between <scene name='84/848919/Hydrophbic_lys19_icat_bcat/3'>the aliphatic portion of the Lys19 side chain and the aromatic rings of Phe660 (ARM 12) and Phe40 (ICAT)</scene> (shown in pink). Lys19 also forms a <scene name='84/848919/Salt_bridge_lys19_icat_bcat/2'>salt bridge</scene> with ß-catenin Glu664 (repeat 12). Another polar interaction occurs between <scene name='84/848919/Arg_glu_icat_bcat/2'>ICAT Glu37 and ß-catenin Arg66</scene>1. <ref name=Daniels & Weis 2002> Daniels, D. L., & Weis, W. I. (2002). ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Molecular cell, 10(3), 573–584. https://doi.org/10.1016/s1097-2765(02)00631-7. </ref> |
[[Image:Canonical Wnt pathway with Wnt..jpg]] | [[Image:Canonical Wnt pathway with Wnt..jpg]] | ||
| - | '''Figure | + | '''Figure 6''': The canonical Wnt pathway when Wnt is present. The inhibition of the destruction complex allows ß-catenin translocation from cytoplasm to nucleus. Source: Gpruett2/CC BY-SA [https://commons.wikimedia.org/wiki/File:Canonical_Wnt_pathway_with_Wnt..jpg] |
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Crystal Structure of a Full-Length Zebrafish Beta-Catenin
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||