User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
< User:Isabela Fonseca de Oliveira Granha(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | = | + | =Crystal Structure of a Full-Length Zebrafish Beta-Catenin= |
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota ([https://en.wikipedia.org/wiki/Eukaryote Eukaryota]) phylum, in humans the gene CTNNB1 ([https://www.ncbi.nlm.nih.gov/gene/1499 CTNNB1]) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway <ref name=logan&nusse2004> DOI : 10.1146/annurev.cellbio.20.010403.113126</ref> (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. <ref name="xing2009">DOI 10.1016/j.str.2007.12.021</ref> | ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota ([https://en.wikipedia.org/wiki/Eukaryote Eukaryota]) phylum, in humans the gene CTNNB1 ([https://www.ncbi.nlm.nih.gov/gene/1499 CTNNB1]) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway <ref name=logan&nusse2004> DOI : 10.1146/annurev.cellbio.20.010403.113126</ref> (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. <ref name="xing2009">DOI 10.1016/j.str.2007.12.021</ref> | ||
| Line 18: | Line 18: | ||
[[Image: Whitebetacatenincartoonhidrophocity.png]] | [[Image: Whitebetacatenincartoonhidrophocity.png]] | ||
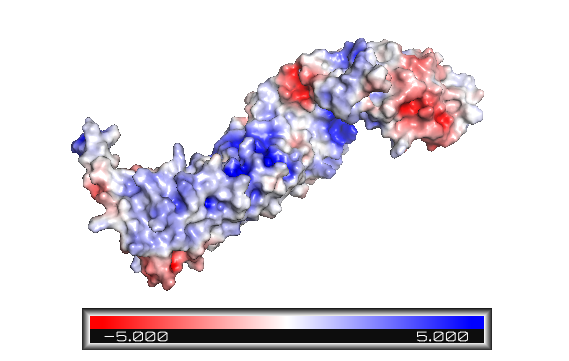
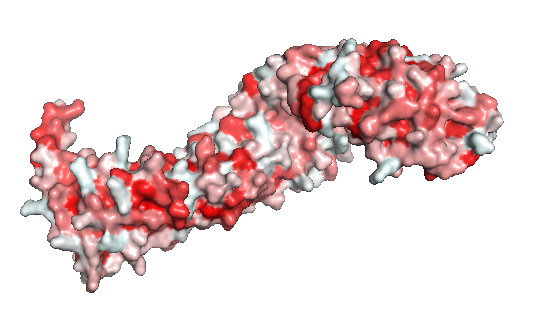
| - | '''Figure 2''': The beta-catenin polarity surface. The red | + | '''Figure 2''': The beta-catenin polarity surface. The red color represents hidrophobic sites, and white, the hidrophilic areas. The protein does not have a partiular polar or apolar area. Polarity is well distributed through the molecule as well as the protein ligands. |
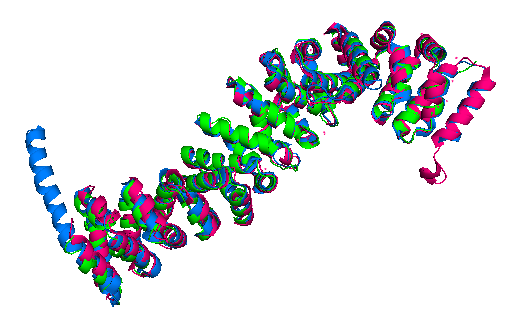
Finally, the ''Danio rerio'' ([http://www.rcsb.org/structure/2Z6G 2Z6G] full length structure, blue), ''Mus musculus'' ([https://www.rcsb.org/structure/2BCT 2BCT] armadillo repeat region, green) and ''Homo sapiens'' ([https://www.rcsb.org/structure/2Z6H 2Z6H] full length structure, pink) beta-catenin alignment (Figure 3) shows that the protein structure is quite similar in these organisms. The three structures have 12 armadillo repeat group and the superposition indicates that the helix C in zebrafish and human beta-catenin conformation and orientation are essentially the same in both crystal structures. This great similarity between these proteins demonstrates that beta-catenin is evolutionary conserved and so are the pathways that it takes part. | Finally, the ''Danio rerio'' ([http://www.rcsb.org/structure/2Z6G 2Z6G] full length structure, blue), ''Mus musculus'' ([https://www.rcsb.org/structure/2BCT 2BCT] armadillo repeat region, green) and ''Homo sapiens'' ([https://www.rcsb.org/structure/2Z6H 2Z6H] full length structure, pink) beta-catenin alignment (Figure 3) shows that the protein structure is quite similar in these organisms. The three structures have 12 armadillo repeat group and the superposition indicates that the helix C in zebrafish and human beta-catenin conformation and orientation are essentially the same in both crystal structures. This great similarity between these proteins demonstrates that beta-catenin is evolutionary conserved and so are the pathways that it takes part. | ||
| Line 31: | Line 31: | ||
[[Image:Beta-catenin-moonlighting.png]] | [[Image:Beta-catenin-moonlighting.png]] | ||
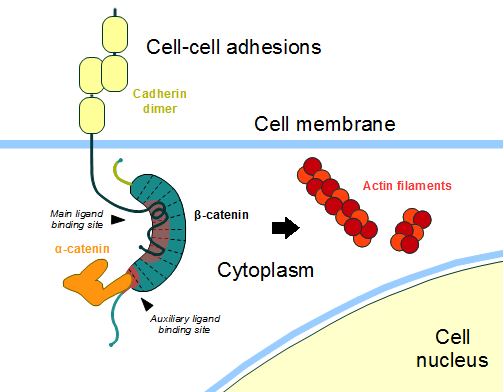
| - | '''Figure 4''': Cadherin-based cell adhesion. Alpha-catenin/ß-catenin forms a heterodimer that can connects to E-cadherin promoting the adherens junctions. As a homodimer, alpha-catenin interacts with actin. Adapted from: Bubus12/CC BY [ | + | '''Figure 4''': Cadherin-based cell adhesion. Alpha-catenin/ß-catenin forms a heterodimer that can connects to E-cadherin promoting the adherens junctions. As a homodimer, alpha-catenin interacts with actin. Adapted from: Bubus12/CC BY [https://commons.wikimedia.org/wiki/File:Beta-catenin-moonlighting.png] |
==The ß-catenin destruction complex== | ==The ß-catenin destruction complex== | ||
| Line 37: | Line 37: | ||
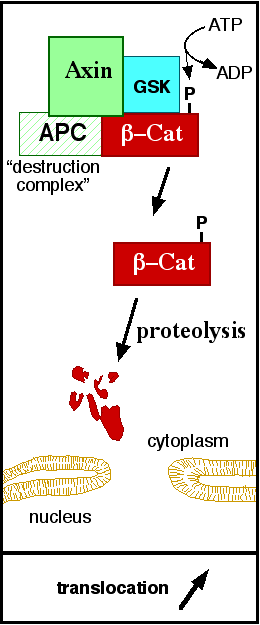
[[Image:Axindestructioncomplex.png]] | [[Image:Axindestructioncomplex.png]] | ||
| - | '''Figure 5''': A simplified diagram of the ß-catenin destruction complex. The destruction complex proteins promote the ß-catenin proteolysis in cytoplasm. Source: JWSchmidt at the English language Wikipedia/CC BY-SA [ | + | '''Figure 5''': A simplified diagram of the ß-catenin destruction complex. The destruction complex proteins promote the ß-catenin proteolysis in cytoplasm. Source: JWSchmidt at the English language Wikipedia/CC BY-SA [https://commons.wikimedia.org/wiki/File:Axindestructioncomplex.png] |
==DNA binding and transcription== | ==DNA binding and transcription== | ||
| Line 46: | Line 46: | ||
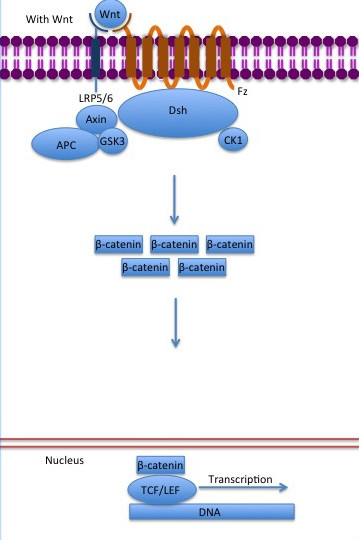
[[Image:Canonical Wnt pathway with Wnt..jpg]] | [[Image:Canonical Wnt pathway with Wnt..jpg]] | ||
| - | '''Figure 6''': The canonical Wnt pathway when Wnt is present. The inhibition of the destruction complex allows ß-catenin translocation from cytoplasm to nucleus. Source: Gpruett2/CC BY-SA [ | + | '''Figure 6''': The canonical Wnt pathway when Wnt is present. The inhibition of the destruction complex allows ß-catenin translocation from cytoplasm to nucleus. Source: Gpruett2/CC BY-SA [https://commons.wikimedia.org/wiki/File:Canonical_Wnt_pathway_with_Wnt..jpg] |
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Crystal Structure of a Full-Length Zebrafish Beta-Catenin
ß-catenin is an important element in cell-cell adherens junctions, called cadherins. Reported in all Eukaryota (Eukaryota) phylum, in humans the gene CTNNB1 (CTNNB1) transcribes a 95kDa protein that allows cadherins to anchor in cytoeskeleton (actin filaments) by connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway [1] (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [2]
| |||||||||||