Journal:Acta Cryst D:S2059798320008116
From Proteopedia
(Difference between revisions)

| Line 7: | Line 7: | ||
This publication describes a high–resolution X-ray crystallographic structure of the Group B Streptococcus BibA N-terminal fragment (BibA126-398), and a low-resolution structure of the full-length N-terminal domain (BibA34-400) determined using small angle X-ray scattering (SAXS). The association of the BibA N-terminal domain was localized to the C4BP α-chain. | This publication describes a high–resolution X-ray crystallographic structure of the Group B Streptococcus BibA N-terminal fragment (BibA126-398), and a low-resolution structure of the full-length N-terminal domain (BibA34-400) determined using small angle X-ray scattering (SAXS). The association of the BibA N-terminal domain was localized to the C4BP α-chain. | ||
| - | <scene name='85/857784/Cv/5'>Cartoon representation of the BibA126–398 crystal structure</scene> shown as a rainbow model with color changing gradually from the N-terminus (blue) to the C-terminus (red). The antiparallel three-helix-bundle-motif repeats are labeled from the N-terminus to the C-terminus as MR1–MR4, respectively. | + | <scene name='85/857784/Cv/5'>Cartoon representation of the BibA126–398 crystal structure</scene> shown as a rainbow model with color changing gradually from the N-terminus (blue) to the C-terminus (red). The antiparallel three-helix-bundle-motif repeats are labeled from the N-terminus to the C-terminus as MR1–MR4, respectively. The overall structure of BibA126–398 has an �150 A ˚ -long distorted rod shape composed of nine �-helices, a 310-helix and small loops connecting the helices. Interestingly, the nine �-helices adopt a unique pattern to form four antiparallel three-helix-bundle-motif repeats labeled MR1 (Asp126– Ser182), MR2 (Thr183–Lys235), MR3 (Glu236–Leu303) and MR4 (Gln304–Asp398) (Fig. 4a). Topologically, the N-terminus first assembles into an 18-residue �-helix (�1; Asp126–Ser142) and a small loop (L1; Leu143–Ser147), followed by the second �-helix (�2; Ser148–Ser160), which runs antiparallel to helix �1. Loop 2 (L2; Ser161–Asp163) proceeds �2, allowing the third �-helix (�3; Ser164–Leu195) to run antiparallel to �2. |
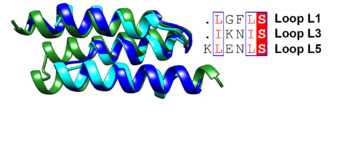
[[Image:Figqqq.png|thumb|350px|left|Superposition of the antiparallel three-helix-bundle-motif repeats MR1 (dark blue), MR2 (cyan) and MR3 (forest green). Similar residues such as Leu and Ile are boxed and shown in a red font and the conserved serine residue is highlighted in red.]] | [[Image:Figqqq.png|thumb|350px|left|Superposition of the antiparallel three-helix-bundle-motif repeats MR1 (dark blue), MR2 (cyan) and MR3 (forest green). Similar residues such as Leu and Ile are boxed and shown in a red font and the conserved serine residue is highlighted in red.]] | ||
Revision as of 13:32, 13 August 2020
α
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.
![(a) Pair distance distribution [P(r)] function of intact BibA34–400 protein. (b) Comparison of the experimental scattering profile (in blue) for BibA34–400 with profiles from a theoretical model (FoXS; green) derived from the proposed BibA34–400 model. (c) Fit of the crystal structure of BibA126–398 (cyan) and the proposed BibA34–400 (magenta) into the ab initio model of BibA34–400 calculated with DAMMIF.](/wiki/images/thumb/9/94/Fig5a-c-relabel-motifs-photoshop-edit.png/500px-Fig5a-c-relabel-motifs-photoshop-edit.png)
