User:Wesley Slaughter/RNA PolII/Sandbox
From Proteopedia
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='1i6h' size='340' side='right' caption='Yeast RNA Polymerase II complex with RNA (PDB code [[1i6h]])'> | |
== Introduction == | == Introduction == | ||
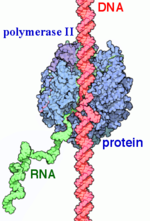
[[ Image:Label RNA pol II (1).png|150px|right|thumb| RNAP II transcription process.]] | [[ Image:Label RNA pol II (1).png|150px|right|thumb| RNAP II transcription process.]] | ||
| Line 21: | Line 21: | ||
=== Pre-Initiation Complex === | === Pre-Initiation Complex === | ||
| - | In both eukaryotes and prokaryotes, the basic mechanism for initiating transcription is the same: protein factors selectively bind to promoter regions on DNA. Prokaryotes use sigma factors while eukaryotes use a complex of 6 GTFs. These GTFs are all named similarly and begin with TF, for transcription factor, followed by the Roman numeral II since they are involved in transcription by RNAP II. The combination of all the transcription factors bound to the DNA promoter region, in complex with RNAP II, is called the PIC. The formation of the PIC occurs in an ordered pathway, beginning with the TATA box which is a promoter region on DNA at position -27. | + | In both eukaryotes and prokaryotes, the basic mechanism for initiating transcription is the same: protein factors selectively bind to promoter regions on DNA. Prokaryotes use sigma factors while eukaryotes use a complex of 6 general transcription factors (GTFs). These GTFs are all named similarly and begin with TF, for transcription factor, followed by the Roman numeral II since they are involved in transcription by RNAP II. The combination of all the transcription factors bound to the DNA promoter region, in complex with RNAP II, is called the pre-initiation complex (PIC). The formation of the PIC occurs in an ordered pathway, beginning with the TATA box which is a promoter region on DNA at position -27. |
| - | Process of PIC formation: | + | Process of PIC formation: L |
1. <scene name='82/824648/Tfiid-tbp/3'>TFIID</scene> contains a subunit named the TATA-binding protein (TBP), which recognizes and binds to the TATA box on the DNA promoter. | 1. <scene name='82/824648/Tfiid-tbp/3'>TFIID</scene> contains a subunit named the TATA-binding protein (TBP), which recognizes and binds to the TATA box on the DNA promoter. | ||
| - | 2. <scene name='82/824648/Tfiib/3'>TFIIB</scene> and <scene name='82/824648/Tfiia/3'>TFIIA</scene> and interact with TBP and are | + | 2. <scene name='82/824648/Tfiib/3'>TFIIB</scene> and <scene name='82/824648/Tfiia/3'>TFIIA</scene> and interact with TBP and are recruited to the promoter. |
3. <scene name='82/824648/Tfiif/5'>TFIIF</scene> binds directly to RNAP II and escorts it to the promoter while TFIIB helps the complex bind correctly. | 3. <scene name='82/824648/Tfiif/5'>TFIIF</scene> binds directly to RNAP II and escorts it to the promoter while TFIIB helps the complex bind correctly. | ||
| - | 4. <scene name='82/824648/Tfiie/4'>TFIIE</scene> and <scene name='82/824648/Tfiih/3'>TFIIH</scene> are | + | 4. <scene name='82/824648/Tfiie/4'>TFIIE</scene> and <scene name='82/824648/Tfiih/3'>TFIIH</scene> are sequentially recruited which completes the <scene name='82/824648/Pic/3'>PIC</scene>. |
| Line 40: | Line 40: | ||
===Initiation=== | ===Initiation=== | ||
| - | + | In the process of initiating transcription, RNAP II recruits several GTFs to bind to the promoter region of the DNA, and this eventually forms the PIC (described above). The DNA enters RNAP II through the clamp, and then it is unwound, creating a <scene name='86/861626/Transcription_bubble_1/2'>transcription bubble</scene>. With the DNA unwound, the <scene name='86/861626/Active_site_initiation/1'>active site</scene> of RNAP II catalyzes the synthesis of the first few RNA bonds. Once the carboxy-terminal domain (CTD) becomes phosphorylated, the clamp undergoes a conformational change to effectively trap the DNA, and a few of the GTFs dissociate, which changes complex to the Elongator complex. | |
===Elongation=== | ===Elongation=== | ||
| Line 75: | Line 75: | ||
Nudler, E. RNA Polymerase Active Center: The Molecular Engine of Transcription. Annu. Rev. Biochem. 2009, 78, 335-361. | Nudler, E. RNA Polymerase Active Center: The Molecular Engine of Transcription. Annu. Rev. Biochem. 2009, 78, 335-361. | ||
| - | Orphanides, George, Thierry Lagrange, and Danny Reinberg. The general transcription factors of RNA polymerase II. Genes & development 10.21. 1996. 2657-2683 | + | L. Orphanides, George, Thierry Lagrange, and Danny Reinberg. The general transcription factors of RNA polymerase II. Genes & development 10.21. 1996. 2657-2683 |
Shah, N. et. al. Tyrosine-1 of RNA Polymerase II CTD Controls Global Termination of Gene Transcription in Mammals. Molecular Cell. 2018, 69, 48-61. | Shah, N. et. al. Tyrosine-1 of RNA Polymerase II CTD Controls Global Termination of Gene Transcription in Mammals. Molecular Cell. 2018, 69, 48-61. | ||
Current revision
| |||||||||||
References
Bushnell, D. A.; Westover, K. D.; Davis, R. E.; Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II-TFIIB Cocrystal at 4.5 Angstroms. Science. 2004, 303, 983-988
Brueckner, F. and Cramer, P. Structural Basis of Transcription Inhibition by -amanitin and Implications for RNA Polymerase II Translocation. Nature Structure and Molecular Biology. 2008, 15, 811-818.
Cramer, P.; Bushnell, D. A.; Kornberg, R. D. Structural Basis of Transcription: RNA Polymerase II at 2.8 Ångstrom Resolution. Science. 2001, 292, 1863-1876
Evans, D. A.; Fitch, D. M.; Smith, T. E.; Cee, V. J. Application of Complex Aldol Reactions to the Total Synthesis of Phorboxazole B. J. Am. Chem. Soc. 2000, 122, 10033-10046.
Gnatt, A. L.; Cramer, P; Fu, J.; Bushnell, D. A.; and Kornberg, R. D. Structural Basis of Transcription: An RNA Polymerase II Elongation Complex at 3.3 Å Resolution. Science. 2001, 292, 1876-1882 1i6h
Hahn, S. Structure and Mechanism of the RNA Polymerase II Transcription Machinery. Nature Structure and Molecular Biology. 2004, 11, 394-403.
He, Yuan, et al. Near-atomic resolution visualization of human transcription promoter opening. Nature 533.7603. 2016.
Nudler, E. RNA Polymerase Active Center: The Molecular Engine of Transcription. Annu. Rev. Biochem. 2009, 78, 335-361.
L. Orphanides, George, Thierry Lagrange, and Danny Reinberg. The general transcription factors of RNA polymerase II. Genes & development 10.21. 1996. 2657-2683
Shah, N. et. al. Tyrosine-1 of RNA Polymerase II CTD Controls Global Termination of Gene Transcription in Mammals. Molecular Cell. 2018, 69, 48-61.
Uzman, A.; Voet, D. Student companion Fundamentals of biochemistry: life at the molecular level, 4th ed., Donald Voet, Judith G. Voet, Charlotte W. Pratt; John Wiley & amp; Sons, 2012.
Xu, J.; Lahiri, I.; Wang, W.; Wier, A.; Cianfrocco, M. A.; Chong, J.; Hare, A. A.; Dervan, P. B.; DiMaio, F.; Leschziner, A. E.; Wang, D. Structural Basis for the Initiation of Eukaryotic Transcription-coupled DNA Repair. Nature. 2017. 551, 653-657 5vvr
Xin, L.; Bushnell, D. A.; and Kornburg, R. D. RNA Polymerase II Transcription: Structure and Mechanism. Biochemica et Biophysica Acta. 2013, 1829, 2-8.
Yan, C., Dodd, T., He, Y., Tainer, J. A., Tsutakawa, S. E., & Ivanov, I. (2019). Transcription preinitiation complex structure and dynamics provide insight into genetic diseases. Nature Structural and Molecular Biology, 26(6), 397-406.
Alpha-aminitin chemical structure image courtesy of https://en.wikipedia.org/wiki/Alpha-Amanitin#/media/File:Alpha-amanitin_structure.png
Notes
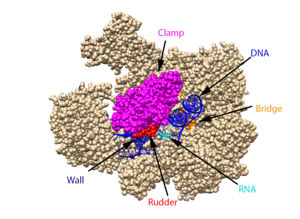
From structural components:
Structural overview: [PDB: 5VVR: with highlighted sections mentioned below]
Bridge: Depicted: [PDB: 1I6H: 810-845.a]
Wall: Depicted: [PDB: 1R5U: 853-919.b; 933-972.b]
Clamp: Depicted: [PDB: 1R5U: 3-345.a; 1395-1435.a; 1158-1124.b]
Rudder: Depicted: [PDB: 5VVR: 306-321.a]
Content Donators
This page was created as a final project for the Advanced Biochemistry course at Wabash College during the Fall of 2019. This page was reviewed by Dr. Wally Novak of Wabash College.