We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
SN2 reaction
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
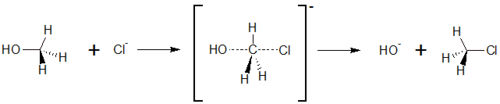
On the other side, SN2 reactions are characterised for exchanging substituents. The substituent that leaves the molecule is called leaving group. | On the other side, SN2 reactions are characterised for exchanging substituents. The substituent that leaves the molecule is called leaving group. | ||
| - | |||
Typically, alkanes with a substituent in primary position undergo S<sub>N</sub>2 reactions. In contrast to a S<sub>N</sub>1 reaction, no stable carbo cation can be formed. Therefore, another way will be taken. | Typically, alkanes with a substituent in primary position undergo S<sub>N</sub>2 reactions. In contrast to a S<sub>N</sub>1 reaction, no stable carbo cation can be formed. Therefore, another way will be taken. | ||
| Line 27: | Line 26: | ||
<jmolButton><script>if(_animating);anim off;else;frame play;endif</script><text>Toggle animation</text></jmolButton> | <jmolButton><script>if(_animating);anim off;else;frame play;endif</script><text>Toggle animation</text></jmolButton> | ||
</jmol> | </jmol> | ||
| - | |||
| - | This demo was adapted from http://www.chemieunterricht-interaktiv.de/en/animations/sn2_substitution/sn2_substitution_3d.html by Dr. V. Pietzner, part of the ChiLe project | ||
===See also=== | ===See also=== | ||
[[SN1_reaction|S<sub>N</sub>1 reaction: Substitution of Cl<sup>−</sup> and ''tert''-Butanol ]] | [[SN1_reaction|S<sub>N</sub>1 reaction: Substitution of Cl<sup>−</sup> and ''tert''-Butanol ]] | ||
| - | </StructureSection> | ||
== References == | == References == | ||
| + | This demo was adapted from http://www.chemieunterricht-interaktiv.de/en/animations/sn2_substitution/sn2_substitution_3d.html by Dr. V. Pietzner, part of the ChiLe project | ||
<references/> | <references/> | ||
| + | </StructureSection> | ||
Revision as of 14:17, 8 October 2020
SN2-Substitution of chloride and methanol
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Jaime Prilusky, Angel Herraez, Verena Pietzner