Staphylococcal nuclease
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
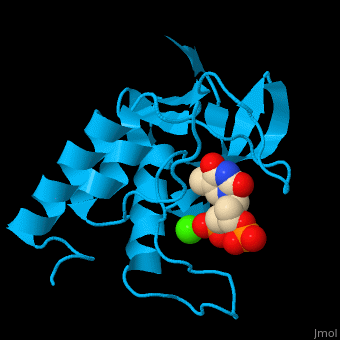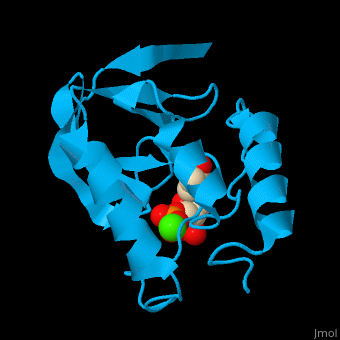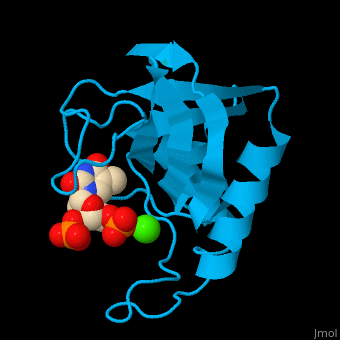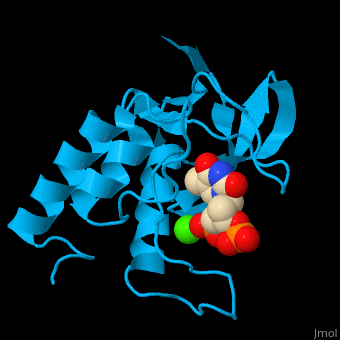
<StructureSection load='' size='350' side='right' scene='49/497241/Cv/1' caption='Stapphylococcal nuclease complex with inhibitor thymidine diphosphate and Ca+2 ion (green) [[1snc]]' pspeed='8'> | <StructureSection load='' size='350' side='right' scene='49/497241/Cv/1' caption='Stapphylococcal nuclease complex with inhibitor thymidine diphosphate and Ca+2 ion (green) [[1snc]]' pspeed='8'> | ||
| - | '''Micrococcal nuclease''', or '''Staphylococcal nuclease''', is a monomeric Ca++ dependent enzyme of 149 amino acids that cleaves either DNA or RNA substrates. It is used for relatively non-specific cleavage of nucleic acids in molecular biology, and has been an important model system for the study of protein folding. | + | '''Micrococcal nuclease''', or '''Staphylococcal nuclease''' or '''Nuclease A''' or '''thermonuclease''', is a monomeric Ca++ dependent enzyme of 149 amino acids that cleaves either DNA or RNA substrates. It is used for relatively non-specific cleavage of nucleic acids in molecular biology, and has been an important model system for the study of protein folding. |
==Structure== | ==Structure== | ||
| Line 7: | Line 7: | ||
*<scene name='49/497241/Cv/7'>Stapphylococcal nuclease interactons with inhibitor thymidine diphosphate (TDP) and Ca+2 ion</scene>. Water molecules are shown as red spheres. | *<scene name='49/497241/Cv/7'>Stapphylococcal nuclease interactons with inhibitor thymidine diphosphate (TDP) and Ca+2 ion</scene>. Water molecules are shown as red spheres. | ||
*<scene name='49/497241/Cv/8'>Ca coordination site</scene>. | *<scene name='49/497241/Cv/8'>Ca coordination site</scene>. | ||
| - | + | ||
==3D structures of Staphylococcal nuclease== | ==3D structures of Staphylococcal nuclease== | ||
| + | [[Staphylococcal nuclease 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==References== | ==References== | ||
Current revision
| |||||||||||
References
- ↑ Arnone AA, Bier CJ, Cotton FA, Day VW, Hazen EE Jr, Richardson DC, Richardson JS, Yonath A (1971) "A High Resolution Structure of an Inhibitor Complex of the Extracellular Nuclease of Staphylococcus aureus: I. Experimental Procedures and Chain Tracing," J. Biol. Chem. 246, 2303-2316
- ↑ Roche J, Caro JA, Norberto DR, Barthe P, Roumestand C, Schlessman JL, Garcia AE, Garcia-Moreno E B, Royer CA. Cavities determine the pressure unfolding of proteins. Proc Natl Acad Sci U S A. 2012 Apr 10. PMID:22496593 doi:10.1073/pnas.1200915109
- ↑ Chen J, Lu Z, Sakon J, Stites WE. Proteins with simplified hydrophobic cores compared to other packing mutants. Biophys Chem. 2004 Aug 1;110(3):239-48. PMID:15228960 doi:10.1016/j.bpc.2004.02.007
- ↑ Wynn R, Harkins PC, Richards FM, Fox RO. Mobile unnatural amino acid side chains in the core of staphylococcal nuclease. Protein Sci. 1996 Jun;5(6):1026-31. PMID:8762134 doi:http://dx.doi.org/10.1002/pro.5560050605
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Jaime Prilusky, Jane S. Richardson