UDP-3-O-acyl-N-acetylglucosamine deacetylase
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
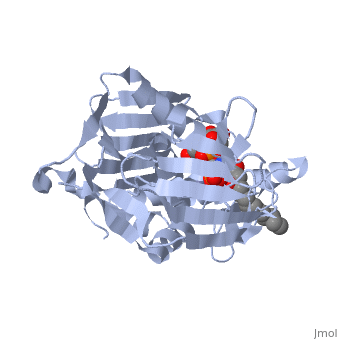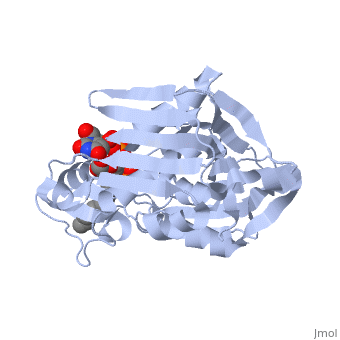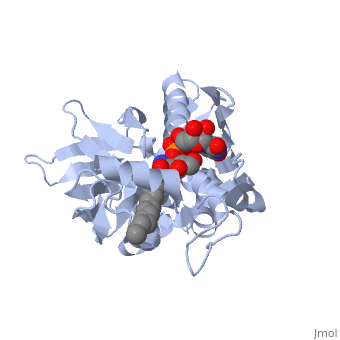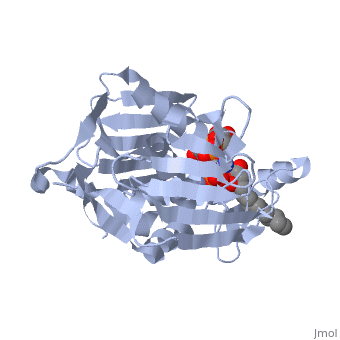
'''UDP-3-O-acyl-N-acetylglucosamine deacetylase''' (LpxC) is a Zn-dependent protein which participates in the biosynthesis of lipid A. Lipid A anchors lipopolysaccharides into the membrane of Gram negative bacteria. LpxC catalyzes the conversion of UDP-3-O-[(3R)-3-hydroxymyristoyl]-N-acetylglucosamine to UDP-3-O-[(3R)-3-hydroxymyristoyl]-N-glucosamine<ref>PMID:18289052</ref>. | '''UDP-3-O-acyl-N-acetylglucosamine deacetylase''' (LpxC) is a Zn-dependent protein which participates in the biosynthesis of lipid A. Lipid A anchors lipopolysaccharides into the membrane of Gram negative bacteria. LpxC catalyzes the conversion of UDP-3-O-[(3R)-3-hydroxymyristoyl]-N-acetylglucosamine to UDP-3-O-[(3R)-3-hydroxymyristoyl]-N-glucosamine<ref>PMID:18289052</ref>. | ||
| - | <scene name='59/595824/Cv/1'>Structure of E. coli UDP-3-O-acyl-N-acetylglucosamine deacetylase complex with Zn+2 ion and UDP-(3-O-(R-3-hydroxymyristoyl))-glucosamine</scene> (PDB code [[4mdt]]). | + | <scene name='59/595824/Cv/1'>Structure of E. coli UDP-3-O-acyl-N-acetylglucosamine deacetylase complex with Zn+2 ion and UDP-(3-O-(R-3-hydroxymyristoyl))-glucosamine</scene> (PDB code [[4mdt]]). |
| + | *<scene name='59/595824/Cv/2'>Zn coordination site</scene>. | ||
Revision as of 14:56, 7 January 2021
| |||||||||||
3D Structures of UDP-3-O-acyl-N-acetylglucosamine deacetylase
Updated on 07-January-2021
References
- ↑ Barb AW, Zhou P. Mechanism and inhibition of LpxC: an essential zinc-dependent deacetylase of bacterial lipid A synthesis. Curr Pharm Biotechnol. 2008 Feb;9(1):9-15. PMID:18289052
- ↑ Pradhan D, Priyadarshini V, Munikumar M, Swargam S, Umamaheswari A, Bitla A. Para-(benzoyl)-phenylalanine as a potential inhibitor against LpxC of Leptospira spp.: homology modeling, docking, and molecular dynamics study. J Biomol Struct Dyn. 2013 Feb 5. PMID:23383626 doi:10.1080/07391102.2012.758056