Ubiquitin-fold modifier
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
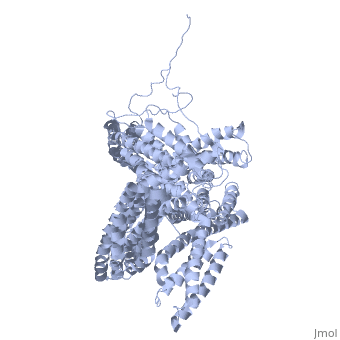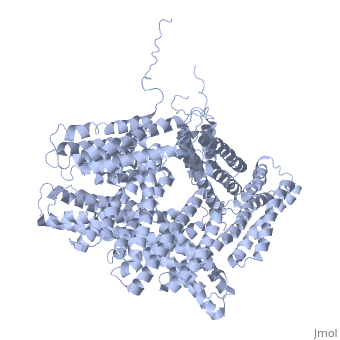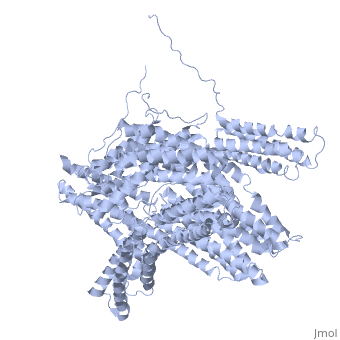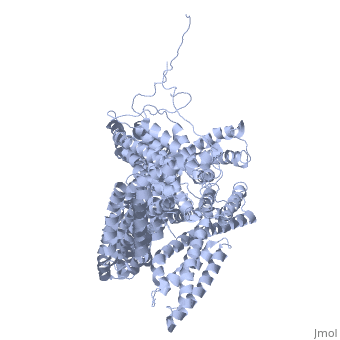
<StructureSection load='1st6' size='340' side='right' caption='Chicken full-length metavinculin, [[1st6]]' scene='' > | <StructureSection load='1st6' size='340' side='right' caption='Chicken full-length metavinculin, [[1st6]]' scene='' > | ||
== Function == | == Function == | ||
| - | '''Ubiquitin-fold modifier''' ( | + | '''Ubiquitin-fold modifier''' (Ufm1) is a member of the ubiquitin-like family. Ufm1 is covalently attached to Lys residues of substrate proteins<ref>PMID:21494687</ref>. This attachment is called Ufmylation and requires '''Ubiquitin-like modifier activating enzyme''' (UBA5) and '''Umf1-conjugating enzyme''' (Ufc1). Ufmylation is a type of post-translational modification that deals with fine-tuned and complex cellular activities and is involved in reticulophagy induced by ER stress<ref>PMID:31129212</ref>. Ufmylation is aribosomal modification specialized to facilitate metazoan-specific protein biogenesis at the ER<ref>PMID:30626644</ref>. |
== Relevance == | == Relevance == | ||
| - | + | Ufm1 participates in maintenance of homeostasis and protection from inflammatory disease<ref>PMID:30701081</ref>. | |
| - | + | ||
| - | + | ||
| - | + | ||
== Structural highlights == | == Structural highlights == | ||
Revision as of 10:50, 3 February 2021
| |||||||||||
3D Structures of Ubiquitin-fold modifier
Updated on 03-February-2021
References
- ↑ Lemaire K, Moura RF, Granvik M, Igoillo-Esteve M, Hohmeier HE, Hendrickx N, Newgard CB, Waelkens E, Cnop M, Schuit F. Ubiquitin fold modifier 1 (UFM1) and its target UFBP1 protect pancreatic beta cells from ER stress-induced apoptosis. PLoS One. 2011 Apr 6;6(4):e18517. doi: 10.1371/journal.pone.0018517. PMID:21494687 doi:http://dx.doi.org/10.1371/journal.pone.0018517
- ↑ Xie Z, Fang Z, Pan Z. Ufl1/RCAD, a Ufm1 E3 ligase, has an intricate connection with ER stress. Int J Biol Macromol. 2019 Aug 15;135:760-767. doi:, 10.1016/j.ijbiomac.2019.05.170. Epub 2019 May 23. PMID:31129212 doi:http://dx.doi.org/10.1016/j.ijbiomac.2019.05.170
- ↑ Walczak CP, Leto DE, Zhang L, Riepe C, Muller RY, DaRosa PA, Ingolia NT, Elias JE, Kopito RR. Ribosomal protein RPL26 is the principal target of UFMylation. Proc Natl Acad Sci U S A. 2019 Jan 22;116(4):1299-1308. doi:, 10.1073/pnas.1816202116. Epub 2019 Jan 9. PMID:30626644 doi:http://dx.doi.org/10.1073/pnas.1816202116
- ↑ Cai Y, Zhu G, Liu S, Pan Z, Quintero M, Poole CJ, Lu C, Zhu H, Islam B, Riggelen JV, Browning D, Liu K, Blumberg R, Singh N, Li H. Indispensable role of the Ubiquitin-fold modifier 1-specific E3 ligase in maintaining intestinal homeostasis and controlling gut inflammation. Cell Discov. 2019 Jan 29;5:7. doi: 10.1038/s41421-018-0070-x. eCollection 2019. PMID:30701081 doi:http://dx.doi.org/10.1038/s41421-018-0070-x