Acylaminoacyl peptidase
From Proteopedia
(Difference between revisions)
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' scene='43/433643/Cv/2' caption='Acylaminoacyl peptidase dimer complex with acetyl and glycerol [[2hu7]]'> |
== Function == | == Function == | ||
| - | [[Acylaminoacyl peptidase]] or Acylamino-acid releasing enzyme ('''AARE''', EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.4.19.1 3.4.19.1]) cleaves N-acetyl or N-formyl amino acid from the N-terminal of polypeptides. | + | [[Acylaminoacyl peptidase]] or '''Acylamino-acid releasing enzyme''' ('''AARE''', EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.4.19.1 3.4.19.1]) cleaves N-acetyl or N-formyl amino acid from the N-terminal of polypeptides.<ref>PMID:21084296</ref> |
== Structural highlights == | == Structural highlights == | ||
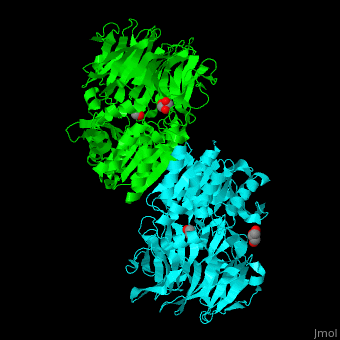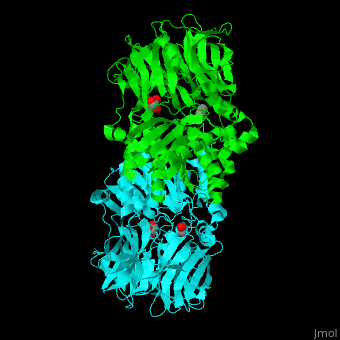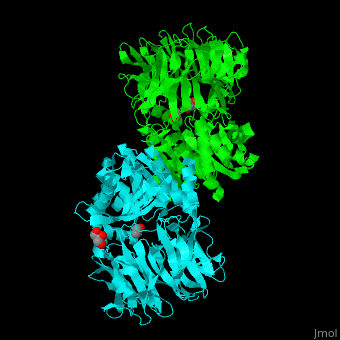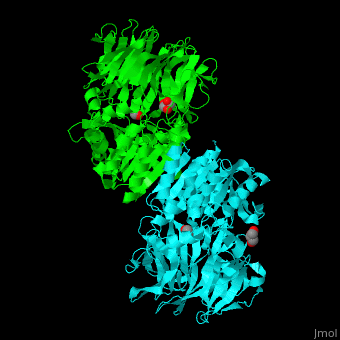
| - | AARE contains a <scene name='43/433643/Cv/ | + | AARE contains a <scene name='43/433643/Cv/6'>C-terminal hydrolase</scene> (in olive) and an <scene name='43/433643/Cv/7'>N-terminal β-propeller domain</scene> (in green). <scene name='43/433643/Cv/8'>Active site is S445, D524, H556</scene>. <ref>PMID:17350041</ref> The enzyme exists in an open state in which the oxyanion active site is accessible to the substrate and in a closed state where the active site is blocked. |
| + | |||
| + | == 3D Structures of Acylaminoacyl peptidase == | ||
| + | [[Acylaminoacyl peptidase 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
__NOTOC__ | __NOTOC__ | ||
| - | == 3D Structures of Acylaminoacyl peptidase == | ||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | |||
| - | [[3o4g]], [[3o4h]], [[3o4i]], [[3o4j]], [[2hu5]], [[2hu7]], [[2hu8]], [[1ve6]] – ApAARE – ''Aeropyrum pernix''<br /> | ||
| - | [[2qr5]], [[2qzp]] – ApAARE (mutant)<br /> | ||
| - | [[1ve7]] – ApAARE+p-nitrophenyl phosphate<br /> | ||
| - | [[3fnb]] – AARE – ''Streptococcus mutans''<br /> | ||
== References == | == References == | ||
| - | + | <references/> | |
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Harmat V, Domokos K, Menyhard DK, Pallo A, Szeltner Z, Szamosi I, Beke-Somfai T, Naray-Szabo G, Polgar L. Structure and catalysis of acylaminoacyl peptidase: closed and open subunits of a dimer oligopeptidase. J Biol Chem. 2010 Nov 16. PMID:21084296 doi:10.1074/jbc.M110.169862
- ↑ Kiss AL, Hornung B, Radi K, Gengeliczki Z, Sztaray B, Juhasz T, Szeltner Z, Harmat V, Polgar L. The acylaminoacyl peptidase from Aeropyrum pernix K1 thought to be an exopeptidase displays endopeptidase activity. J Mol Biol. 2007 Apr 27;368(2):509-20. Epub 2007 Feb 20. PMID:17350041 doi:10.1016/j.jmb.2007.02.025