User:Betsy Johns/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
==== Acyl-CoA Binding ==== | ==== Acyl-CoA Binding ==== | ||
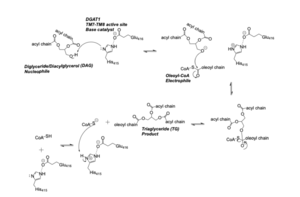
<scene name='87/877515/Acyl_coa/4'>Acyl-CoA</scene> enters DGAT’s active site through a channel on the cytosolic side of the membrane. In order for the channel to accommodate the fatty acid tail of the Acyl-CoA, His415 must first break its interactions with Met434. This allows for the His415 to swing down and have hydrogen bond interactions with Gln465, thus widening the channel enough for the fatty acid tail to move into the active site. Once within the active site, residues Asn378, Gln437, Met434, His415, and Gln465 directly interact with and stabilize the fatty acid tail within the cytosolic channel of the active site. | <scene name='87/877515/Acyl_coa/4'>Acyl-CoA</scene> enters DGAT’s active site through a channel on the cytosolic side of the membrane. In order for the channel to accommodate the fatty acid tail of the Acyl-CoA, His415 must first break its interactions with Met434. This allows for the His415 to swing down and have hydrogen bond interactions with Gln465, thus widening the channel enough for the fatty acid tail to move into the active site. Once within the active site, residues Asn378, Gln437, Met434, His415, and Gln465 directly interact with and stabilize the fatty acid tail within the cytosolic channel of the active site. | ||
| + | <scene name='87/877509/Surface_active_site/3'>Acyl CoA bound in active site</scene> | ||
== Disease == | == Disease == | ||
Revision as of 18:47, 5 April 2021
Diacylglycerol acyltransferase, DGAT
| |||||||||||
References
- ↑ 1.0 1.1 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ 2.0 2.1 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
Student Contributors
- Betsy Johns
- Elise Wang
- Tyler Bihasa