Calmodulin
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
== Calmodulin in Motion == | == Calmodulin in Motion == | ||
| + | The buttons below allow you to explore a morph between structures [[1prw]] and [[1cll]]. | ||
<jmol> | <jmol> | ||
<jmolButton> | <jmolButton> | ||
| Line 64: | Line 65: | ||
</jmol> | </jmol> | ||
| + | The following morph is between [[1prw]] and [[1cll]] after superposition of residues 79-138. This shows the subtle conformational changes in that domain more clearly. | ||
| + | <jmol> | ||
| + | <jmolButton> | ||
| + | <script> | ||
| + | script "/wiki/images/1/1a/Morph.spt"; | ||
| + | load files "=1prw" "=1cll"; | ||
| + | delete water; | ||
| + | delete protein and not backbone; | ||
| + | select all;cartoon only;cartoon off; | ||
| + | select 4-147;cartoon on; | ||
| + | model 1; | ||
| + | center visible;color group; | ||
| + | compare {2.1} {1.1} SUBSET{*.CA} ATOMS{79-138}{79-138} ROTATE TRANSLATE; | ||
| + | </script> | ||
| + | <text>Prepare Animation</text> | ||
| + | </jmolButton> | ||
| + | </jmol><jmol> | ||
| + | <jmolButton> | ||
| + | <script> | ||
| + | model 2; | ||
| + | display 4-147; | ||
| + | backbone only; | ||
| + | backbone 0.5 | ||
| + | structures = [{1.1}, {2.1}]; | ||
| + | my_recipe = [ | ||
| + | [0, 1, 0, {79-147},{79-147}, {(79-138) and alpha}, 0], | ||
| + | [0, 1, 0, {4-78}, {79}, {(8-78) and alpha}, 0], | ||
| + | ]; | ||
| + | morph(20, structures, my_recipe, 0); | ||
| + | </script> | ||
| + | <text>Open</text> | ||
| + | </jmolButton> | ||
| + | </jmol><jmol> | ||
| + | <jmolButton> | ||
| + | <script> | ||
| + | model 1 | ||
| + | display 4-147; | ||
| + | backbone only; | ||
| + | backbone 0.5 | ||
| + | structures = [{2.1}, {1.1}]; | ||
| + | my_recipe = [ | ||
| + | [0, 1, 0, {79-147},{79-147}, {(79-138) and alpha}, 0], | ||
| + | [0, 1, 0, {4-78}, {79}, {(8-78) and alpha}, 0], | ||
| + | ]; | ||
| + | morph(20, structures, my_recipe, 0); | ||
| + | </script> | ||
| + | <text>Close</text> | ||
| + | </jmolButton> | ||
| + | </jmol> | ||
| + | |||
| + | The following morph is between [[1prw]] and [[1cll]] after superposition of residues 8-78. This shows the subtle conformational changes in that domain more clearly. | ||
| + | <jmol> | ||
| + | <jmolButton> | ||
| + | <script> | ||
| + | script "/wiki/images/1/1a/Morph.spt"; | ||
| + | load files "=1prw" "=1cll"; | ||
| + | delete water; | ||
| + | delete protein and not backbone; | ||
| + | select all;cartoon only;cartoon off; | ||
| + | select 4-147;cartoon on; | ||
| + | model 1; | ||
| + | center visible;color group; | ||
| + | compare {2.1} {1.1} SUBSET{*.CA} ATOMS{8-78}{8-78} ROTATE TRANSLATE; | ||
| + | </script> | ||
| + | <text>Prepare Animation</text> | ||
| + | </jmolButton> | ||
| + | </jmol><jmol> | ||
| + | <jmolButton> | ||
| + | <script> | ||
| + | model 2; | ||
| + | display 4-147; | ||
| + | backbone only; | ||
| + | backbone 0.5 | ||
| + | structures = [{1.1}, {2.1}]; | ||
| + | my_recipe = [ | ||
| + | [0, 1, 0, {4-78}, {4-78}, {(8-78) and alpha}, 0], | ||
| + | [0, 1, 0, {79-147},{78}, {(79-138) and alpha}, 0], | ||
| + | ]; | ||
| + | morph(20, structures, my_recipe, 0); | ||
| + | </script> | ||
| + | <text>Open</text> | ||
| + | </jmolButton> | ||
| + | </jmol><jmol> | ||
| + | <jmolButton> | ||
| + | <script> | ||
| + | model 1 | ||
| + | display 4-147; | ||
| + | backbone only; | ||
| + | backbone 0.5 | ||
| + | structures = [{2.1}, {1.1}]; | ||
| + | my_recipe = [ | ||
| + | [0, 1, 0, {4-78}, {4-78}, {(8-78) and alpha}, 0], | ||
| + | [0, 1, 0, {79-147},{78}, {(79-138) and alpha}, 0], | ||
| + | ]; | ||
| + | morph(20, structures, my_recipe, 0); | ||
| + | </script> | ||
| + | <text>Close</text> | ||
| + | </jmolButton> | ||
| + | </jmol> | ||
The clip represents Calmodulin in motion. At the beginning it is shown moving in the unbound form (ApoCaM), and it changes its conformation when Calcium ions are present in the medium (CaCaM). | The clip represents Calmodulin in motion. At the beginning it is shown moving in the unbound form (ApoCaM), and it changes its conformation when Calcium ions are present in the medium (CaCaM). | ||
Revision as of 21:27, 10 April 2021
| |||||||||||
See Also
Bibliography
- ↑ Chattopadhyaya R, Meador WE, Means AR, Quiocho FA. Calmodulin structure refined at 1.7 A resolution. J Mol Biol. 1992 Dec 20;228(4):1177-92. PMID:1474585
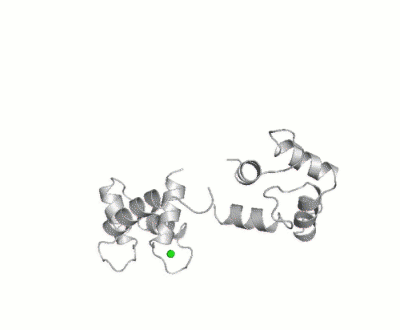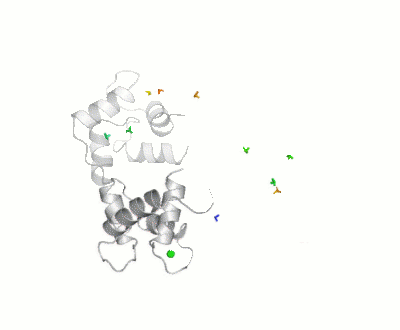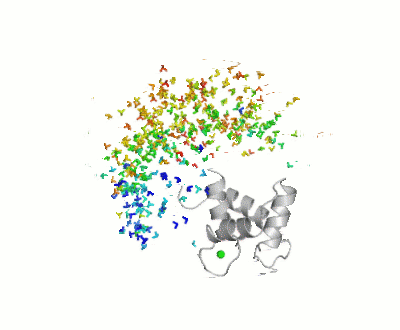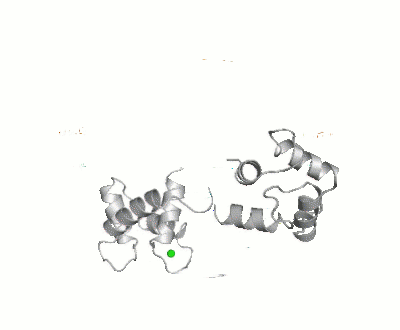
- ↑ Bertini I, Giachetti A, Luchinat C, Parigi G, Petoukhov MV, Pierattelli R, Ravera E, Svergun DI. Conformational Space of Flexible Biological Macromolecules from Average Data. J Am Chem Soc. 2010 Sep 7. PMID:20822180 doi:10.1021/ja1063923
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Karsten Theis, Jaime Prilusky, Enrico Ravera, Daniel Moyano-Marino