This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Megan Leaman/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
=== Mechanism === | === Mechanism === | ||
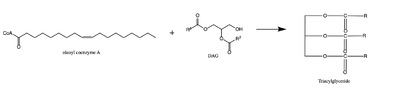
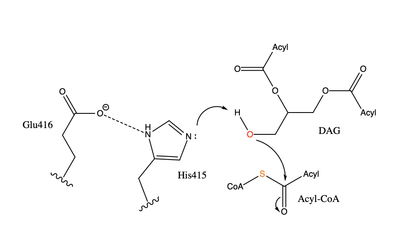
| - | [[Image:dgat chemdraw mechanism.png|400 px|right|thumb|Figure 4: Arrow pushing mechanism of DGAT1 triglyceride synthesis]] When a molecule of diacylglycerol (DAG), or another acyl acceptor, binds into this hydrophobic tunnel, DGAT1 transfers the acyl group on the bound oleoyl-CoA to the DAG to form a triglyceride (Fig. 1). The <scene name='87/878228/415_416_465_with_oleoyl_coa/2'>Catalytic His415</scene> deprotonates the hydroxyl group on the C3 of the glycerol backbone. The deprotonated oxygen then makes a nucleophilic attack on the carbonyl carbon of the Acyl-CoA, the electron density gets shifted up to the oxygen and the tetrahedral acyl-CoA-DAG intermediate is formed which is likely stabilized by Gln465. <ref name="Wang">PMID:32433610</ref>The electron density then falls back down to the carbonyl carbon and to the sulfur of the Acyl-CoA which accepts the added electron density and the bond between the sulfur and carbonyl carbon is broken. Glu416 likely provides enhancement of the reaction by deprotonating His415 which shifts the electron density and helps facilitate the deprotonation by His415 of the glycerol backbone despite being outside of the 3 angstrom hydrogen bonding distance. This is likely due to the fact that the DGAT 1 enzyme was unable to be visualized with the diacylglycerol in the active site. The entrance and binding of the diacylglycerol may cause conformational changes and shifting of the Glu416 to become closer to the His415. Point mutations made to His415 and Glu416 support the hypothesis that it is essential for catalysis in the active site since the enzyme function was completely eliminated when the mutation was made. <ref name="Wang">PMID:32433610</ref> | + | [[Image:dgat chemdraw mechanism.png|400 px|right|thumb|Figure 4: Arrow pushing mechanism of DGAT1 triglyceride synthesis]] When a molecule of diacylglycerol (DAG), or another acyl acceptor, binds into this hydrophobic tunnel, DGAT1 transfers the acyl group on the bound oleoyl-CoA to the DAG to form a triglyceride (Fig. 1). The <scene name='87/878228/415_416_465_with_oleoyl_coa/2'>Catalytic His415</scene> deprotonates the hydroxyl group on the C3 of the glycerol backbone. The deprotonated oxygen then makes a nucleophilic attack on the carbonyl carbon of the Acyl-CoA, the electron density gets shifted up to the oxygen and the tetrahedral acyl-CoA-DAG intermediate is formed which is likely stabilized by Gln465. <ref name="Wang">PMID:32433610</ref> The electron density then falls back down to the carbonyl carbon and to the sulfur of the Acyl-CoA which accepts the added electron density and the bond between the sulfur and carbonyl carbon is broken. Glu416 likely provides enhancement of the reaction by deprotonating His415 which shifts the electron density and helps facilitate the deprotonation by His415 of the glycerol backbone despite being outside of the 3 angstrom hydrogen bonding distance. This is likely due to the fact that the DGAT 1 enzyme was unable to be visualized with the diacylglycerol in the active site. The entrance and binding of the diacylglycerol may cause conformational changes and shifting of the Glu416 to become closer to the His415. Point mutations made to His415 and Glu416 support the hypothesis that it is essential for catalysis in the active site since the enzyme function was completely eliminated when the mutation was made. <ref name="Wang">PMID:32433610</ref> |
===Inhibitors=== | ===Inhibitors=== | ||
Revision as of 03:00, 23 April 2021
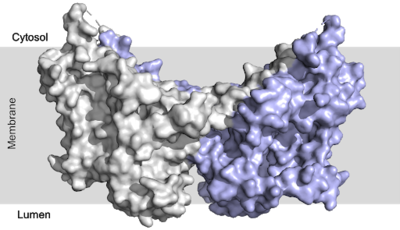
Human Diacylglycerol O-Transferase 1
| |||||||||||
References
- ↑ 1.0 1.1 Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, Novak S, Collins C, Welch CB, Lusis AJ, Erickson SK, Farese RV Jr. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13018-23. PMID:9789033
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
- ↑ 3.0 3.1 Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV Jr. Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008 Nov;49(11):2283-301. doi: 10.1194/jlr.R800018-JLR200. Epub 2008, Aug 29. PMID:18757836 doi:http://dx.doi.org/10.1194/jlr.R800018-JLR200
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ Haas, J. T., Winter, H. S., Lim, E., Kirby, A., Blumenstiel, B., DeFelice, M., Gabriel, S., Jalas, C., Branski, D., Grueter, C. A., Toporovski, M. S., Walther, T. C., Daly, M. J., & Farese, R. V., Jr (2012). DGAT1 mutation is linked to a congenital diarrheal disorder. The Journal of clinical investigation, 122(12), 4680–4684. https://doi.org/10.1172/JCI64873
- ↑ 6.0 6.1 Gluchowski, N. L., Chitraju, C., Picoraro, J. A., Mejhert, N., Pinto, S., Xin, W., Kamin, D. S., Winter, H. S., Chung, W. K., Walther, T. C., & Farese, R. V., Jr (2017). Identification and characterization of a novel DGAT1 missense mutation associated with congenital diarrhea. Journal of lipid research, 58(6), 1230–1237. https://doi.org/10.1194/jlr.P075119
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
Student Contributors
- Megan Leaman
- Grace Hall
- Karina Latsko