Sandbox GGC9
From Proteopedia
| Line 24: | Line 24: | ||
| - | Recent studies identified aspartic acid residues at positions 600 and 708 function to initiate catalysis. | + | Recent studies identified aspartic acid residues at positions 600 and 708 function to initiate catalysis.[7] |
<scene name='75/752271/Catalytic_residues/1'>Catalytic Residues</scene> | <scene name='75/752271/Catalytic_residues/1'>Catalytic Residues</scene> | ||
| Line 38: | Line 38: | ||
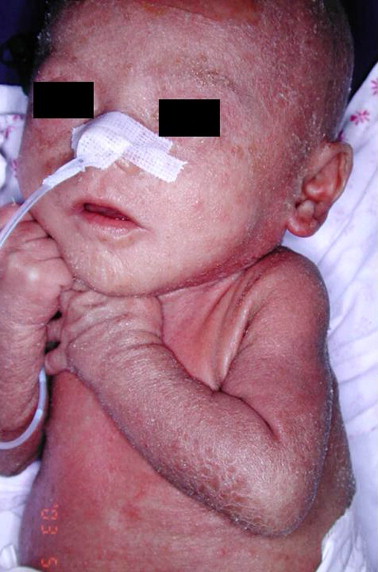
[4] Omenn syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. Rarediseases.info.nih.gov. (2021). Retrieved 7 April 2021, from https://rarediseases.info.nih.gov/diseases/8198/omenn-syndrome. | [4] Omenn syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. Rarediseases.info.nih.gov. (2021). Retrieved 7 April 2021, from https://rarediseases.info.nih.gov/diseases/8198/omenn-syndrome. | ||
| - | |||
[5] Gwyn, Lori M et al. “A zinc site in the C-terminal domain of RAG1 is essential for DNA cleavage activity.” Journal of molecular biology vol. 390,5 (2009): 863-78. doi:10.1016/j.jmb.2009.05.076 | [5] Gwyn, Lori M et al. “A zinc site in the C-terminal domain of RAG1 is essential for DNA cleavage activity.” Journal of molecular biology vol. 390,5 (2009): 863-78. doi:10.1016/j.jmb.2009.05.076 | ||
[6] Hsu, C., Yu-Yun Lee, J., & Chao, S. (2011). Omenn syndrome: a case report and review of literature. Dermatologica Sinica, 29(2). https://doi.org/doi.org/10.1016/j.dsi.2011.05.002 | [6] Hsu, C., Yu-Yun Lee, J., & Chao, S. (2011). Omenn syndrome: a case report and review of literature. Dermatologica Sinica, 29(2). https://doi.org/doi.org/10.1016/j.dsi.2011.05.002 | ||
| + | |||
| + | [7] Fugmann, S., Villey, I., Ptaszek, L., & Schatz, D. (2000). Identification of Two Catalytic Residues in RAG1 that Define a Single Active Site within the RAG1/RAG2 Protein Complex. Molecular Cell, 5(1), 97-107. https://doi.org/10.1016/s1097-2765(00)80406-2 | ||
Revision as of 02:45, 26 April 2021
Structure of RAG1/2-DNA Strand Transfer Complex (paired conformation)
| |||||||||||
References
[1] Grazini U, Zanardi F, Citterio E, Casola S, Goding CR, McBlane F. The RING domain of RAG1 ubiquitylates histone H3: a novel activity in chromatin-mediated regulation of V(D)J joining. Mol Cell. 2010 Jan 29;37(2):282-93. doi: 10.1016/j.molcel.2009.12.035. PMID: 20122409.
[2] Zhang Y, Corbett E, Wu S, Schatz DG. Structural basis for the activation and suppression of transposition during evolution of the RAG recombinase. EMBO J. 2020 Nov 2;39(21):e105857. doi: 10.15252/embj.2020105857. Epub 2020 Sep 18. PMID: 32945578; PMCID: PMC7604617.
[3] Chen, Karin et al. “Autoimmunity due to RAG deficiency and estimated disease incidence in RAG1/2 mutations.” The Journal of allergy and clinical immunology vol. 133,3 (2014): 880-2.e10. doi:10.1016/j.jaci.2013.11.038
[4] Omenn syndrome | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program. Rarediseases.info.nih.gov. (2021). Retrieved 7 April 2021, from https://rarediseases.info.nih.gov/diseases/8198/omenn-syndrome.
[5] Gwyn, Lori M et al. “A zinc site in the C-terminal domain of RAG1 is essential for DNA cleavage activity.” Journal of molecular biology vol. 390,5 (2009): 863-78. doi:10.1016/j.jmb.2009.05.076
[6] Hsu, C., Yu-Yun Lee, J., & Chao, S. (2011). Omenn syndrome: a case report and review of literature. Dermatologica Sinica, 29(2). https://doi.org/doi.org/10.1016/j.dsi.2011.05.002
[7] Fugmann, S., Villey, I., Ptaszek, L., & Schatz, D. (2000). Identification of Two Catalytic Residues in RAG1 that Define a Single Active Site within the RAG1/RAG2 Protein Complex. Molecular Cell, 5(1), 97-107. https://doi.org/10.1016/s1097-2765(00)80406-2