We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
2ada
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='2ada' size='340' side='right'caption='[[2ada]], [[Resolution|resolution]] 2.40Å' scene=''> | <StructureSection load='2ada' size='340' side='right'caption='[[2ada]], [[Resolution|resolution]] 2.40Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
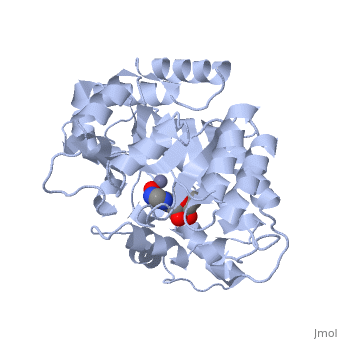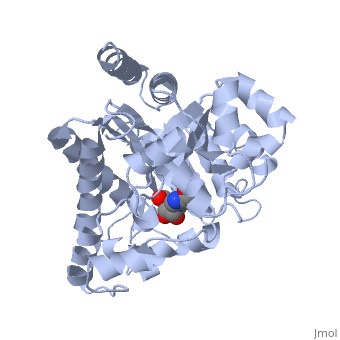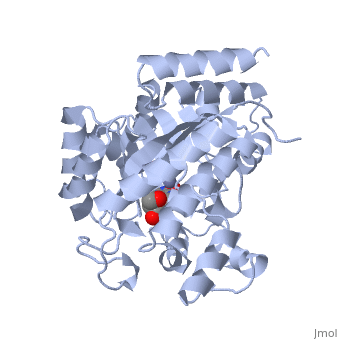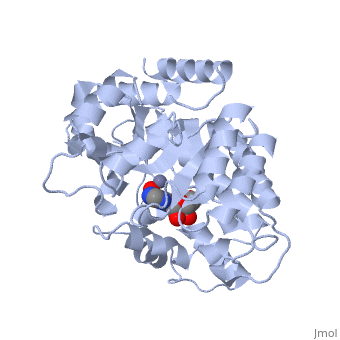
| - | <table><tr><td colspan='2'>[[2ada]] is a 1 chain structure with sequence from [ | + | <table><tr><td colspan='2'>[[2ada]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Lk3_transgenic_mice Lk3 transgenic mice]. This structure supersedes the now removed PDB entry [http://oca.weizmann.ac.il/oca-bin/send-pdb?obs=1&id=1ada 1ada]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2ADA OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2ADA FirstGlance]. <br> |
| - | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=HPR:6-HYDROXY-7,8-DIHYDRO+PURINE+NUCLEOSIDE'>HPR</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> | + | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=HPR:6-HYDROXY-7,8-DIHYDRO+PURINE+NUCLEOSIDE'>HPR</scene>, <scene name='pdbligand=ZN:ZINC+ION'>ZN</scene></td></tr> |
| - | <tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | <tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[https://en.wikipedia.org/wiki/Adenosine_deaminase Adenosine deaminase], with EC number [https://www.brenda-enzymes.info/php/result_flat.php4?ecno=3.5.4.4 3.5.4.4] </span></td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2ada FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2ada OCA], [https://pdbe.org/2ada PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2ada RCSB], [https://www.ebi.ac.uk/pdbsum/2ada PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2ada ProSAT]</span></td></tr> |
</table> | </table> | ||
== Function == | == Function == | ||
| - | [[ | + | [[https://www.uniprot.org/uniprot/ADA_MOUSE ADA_MOUSE]] Catalyzes the hydrolytic deamination of adenosine and 2-deoxyadenosine. Plays an important role in purine metabolism and in adenosine homeostasis. Modulates signaling by extracellular adenosine, and so contributes indirectly to cellular signaling events. Acts as a positive regulator of T-cell coactivation, by binding DPP4. Its interaction with DPP4 regulates lymphocyte-epithelial cell adhesion (By similarity). |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
Revision as of 14:39, 17 November 2021
ATOMIC STRUCTURE OF ADENOSINE DEAMINASE COMPLEXED WITH A TRANSITION-STATE ANALOG: UNDERSTANDING CATALYSIS AND IMMUNODEFICIENCY MUTATIONS
| |||||||||||