Organic Hydroperoxide Resistance Protein from Xylella fastidiosa (Ohr)
OhrR plays a critical role in sensing of reactive oxygen species in the pathogenic bacteria Xanthomonas campestris. Under normal cellular conditions, dimeric OhrR protein is bound to DNA to repress expression of ohr, which encodes organic hydroperoxide resistance protein. When the reactive oxygen species organic hydroperoxide (OHR) is present in the cell, it oxidizes a reactive cysteine in OhrR, resulting in a conformational change that causes the dissocation of OhrR from ohr and subsequent clearance of OHR by OHR. This dissociation occurs because the oxidation of this reactive cysteine results in the formation of an interprotein cysteine bond between each of two units.
Organic Hydroperoxide Resistance Protein from Xylella fastidiosa (Ohr)
OhrR plays a critical role in sensing of reactive oxygen species in the pathogenic bacteria Xanthomonas campestris. Under normal cellular conditions, dimeric OhrR protein is bound to DNA to repress expression of ohr, which encodes organic hydroperoxide resistance protein. When the reactive oxygen species organic hydroperoxide (OHR) is present in the cell, it oxidizes a reactive cysteine in OhrR, resulting in a conformational change that causes the dissocation of OhrR from ohr and subsequent clearance of OHR by OHR. This dissociation occurs because the oxidation of this reactive cysteine results in the formation of an interprotein cysteine bond between each of two units.
Ohr gene regulation
Newbery et al 2007 reported the first crystal structure of OhrR from Xanthomonas campestris in Molecular Cell. This was the second structure of an OhrR protein to be submitted to the Protein Data Bank. For the structures of both reduced and oxidized OhrR, protein was overexpressed in E coli. To produce crystals of the reduced form of the protein, site-directed mutagenesis was performed to mutate the reactive cysteine (Cys22) to a serine. Crystals of both unlabeled and selenomethionine-substituted reduced OhrR were generated and data collected using SAD phasing. For crystallization of the oxidized form of the protein, purified protein was treated with cumene hydroperoxide and purified via gel filtration prior to crystallization. The resulting reduced and oxidized structures were respectively named 2pex and 2pfb. Refinement of 2pex resulted in a 1.90 angstrom structure with an Rfree of 27.7% and Rwork of 23.6% and 96.7% of phi,psi angles in the most favorable regions of the Ramachandran plot. Refinement of 2pfb yielded a 1.93 angstrom structure with 96.1% of phi,psi angles in the most favorable regions of the Ramachandran plot and Rfree/Rwork value of 25.0% and 21.9% respectively. Neither of the final models included any residues in disallowed regions of the Ramachandran plot.
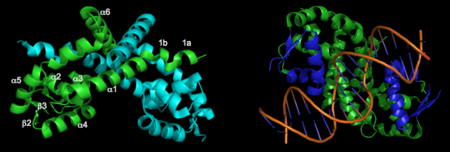
Structural features
The resulting model 2pex shows the biologically relevant dimer of the protein. Each subunit of the dimer is composed of six α-helices and 3 β-strands, as indicated below (left). The specific residues corresponding to these regions of secondary structure are as follows: α1 (residues 21–39), α2 (residues 47–58), β1(residues 62–63), α3 (residues 64–71), α4 (residues 75–87), β2 (residues 91–94), β3 (residues 104–107), α5 (residues 109–129), α6 (residues 133–151), and three-ten helices 1a (residues 13–15) and 1b (residues 17–19). The longest α-helix, α5, has a notable kink at residue G119. The dimerization interface is largely formed by three-ten helices (1a, 1b) and α1, α5, and α6 from each subunit. The extensive dimerization domain buries 5391Ų.
3D structures of Ohr
Organic hydroperoxide resistance protein

