Introduction
Ohr é uma enzima antioxidante dependente de tiol que pertence à família das Ohr/OsmC, presente em bactérias e fungos, e que tem por função a proteção destes microrganismos ao estresse oxidativo. É evidente, portanto, que essa proteína tem um papel central na defesa bacteriana contra oxidantes advindos da reação do hospedeiro à infecção. Com o avanço dos estudos a respeito desse composto descobriu-se que as Ohr são os atores essenciais na decomposição de hidroperóxidos de ácidos graxos e peroxinitrito, ou seja, possui esses compostos como seu principal substrato, não apresentando muita eficiência na decomposição de outros tipos de oxidantes. Dentro desse contexto é importante salientar que apesar de semelhantes às peroxirredoxinas, as Ohr não são vistas como tal. As peroxirredoxinas nada mais são que proteínas que também possuem ação antioxidante mas que tem uma estrutura tanto primária quanto terciária, bem diferente das enzimas Ohr. Um outro ponto importante a se levantar é a relevância dos estudos das Ohr para a criação de novos fármacos, visto que é uma enzima cuja estrutura é característica apenas a bactérias e fungos, não apresentando estrutura similar em animais e plantas. Alguns exemplos de bactérias patogênicas que expressam proteínas Ohr são Pseudomonas aeruginosa, Vibrio cholerae e Xylella fastidiosa. É importante salientar que essa proteína varia de acordo com o microrganismo estudado, tendo diferenças em sua regulação e expressão, sendo o exemplo usado para essa página a proteína Ohr de Xylella fastidiosa
Organic Hydroperoxide Resistance Protein from Xylella fastidiosa (Ohr)
Xylella fastidiosa é uma bactéria gram negativa, colonizadora restrita do xilema de plantas, conhecida por causar doenças em diversas monocotiledôneas e dicotiledôneas de grande importância econômica. Abordando a relação patógeno-hospedeiro foi possível observar que a planta libera espécies reativas de oxigênios que funcionam como agentes microbicidas. Dentre as ROS produzidas se encontram os hidroperóxidos de ácidos graxos, gerados a partir de uma reação de oxidação catalisada pelas enzimas lipoxigenases. Para se defender desse ataque oxidativo a Xylella produz a enzima Ohr. Dentro desse tópico foi observado que em uma situação de aumento do estresse não há aumento da quantidade de enzima, concluindo-se que nessa bactéria não há um mecanismo regulatório como para a maioria dos MO que possuem o gene da Ohr.
Ohr gene regulation
Newbery et al 2007 reported the first crystal structure of OhrR from Xanthomonas campestris in Molecular Cell. This was the second structure of an OhrR protein to be submitted to the Protein Data Bank. For the structures of both reduced and oxidized OhrR, protein was overexpressed in E coli. To produce crystals of the reduced form of the protein, site-directed mutagenesis was performed to mutate the reactive cysteine (Cys22) to a serine. Crystals of both unlabeled and selenomethionine-substituted reduced OhrR were generated and data collected using SAD phasing. For crystallization of the oxidized form of the protein, purified protein was treated with cumene hydroperoxide and purified via gel filtration prior to crystallization. The resulting reduced and oxidized structures were respectively named 2pex and 2pfb. Refinement of 2pex resulted in a 1.90 angstrom structure with an Rfree of 27.7% and Rwork of 23.6% and 96.7% of phi,psi angles in the most favorable regions of the Ramachandran plot. Refinement of 2pfb yielded a 1.93 angstrom structure with 96.1% of phi,psi angles in the most favorable regions of the Ramachandran plot and Rfree/Rwork value of 25.0% and 21.9% respectively. Neither of the final models included any residues in disallowed regions of the Ramachandran plot.
Structural features
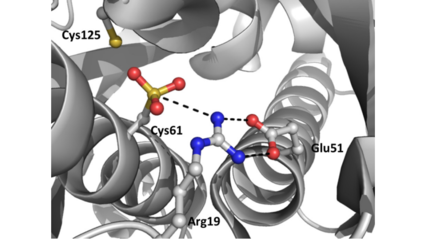
The resulting model 2pex shows the biologically relevant dimer of the protein. Each subunit of the dimer is composed of six α-helices and 3 β-strands, as indicated below (left). The specific residues corresponding to these regions of secondary structure are as follows: α1 (residues 21–39), α2 (residues 47–58), β1(residues 62–63), α3 (residues 64–71), α4 (residues 75–87), β2 (residues 91–94), β3 (residues 104–107), α5 (residues 109–129), α6 (residues 133–151), and three-ten helices 1a (residues 13–15) and 1b (residues 17–19). The longest α-helix, α5, has a notable kink at residue G119. The dimerization interface is largely formed by three-ten helices (1a, 1b) and α1, α5, and α6 from each subunit. The extensive dimerization domain buries 5391Ų.
3D structures of Ohr
Organic hydroperoxide resistance protein