Journal:Acta Cryst F:S2053230X21013455
From Proteopedia
(Difference between revisions)

| Line 7: | Line 7: | ||
*<scene name='89/899476/Cv/2'>Monomer of apo BpBADH</scene>, colored in rainbow from blue (N-terminus) to red (C-terminus); [[6wsa]]. | *<scene name='89/899476/Cv/2'>Monomer of apo BpBADH</scene>, colored in rainbow from blue (N-terminus) to red (C-terminus); [[6wsa]]. | ||
*<scene name='89/899476/Cv/3'>Dimer of BpBADH with NAD</scene>, monomers are shown as green and cyan ribbons, with NAD shown as ball-and-sticks; [[6wsb]]. | *<scene name='89/899476/Cv/3'>Dimer of BpBADH with NAD</scene>, monomers are shown as green and cyan ribbons, with NAD shown as ball-and-sticks; [[6wsb]]. | ||
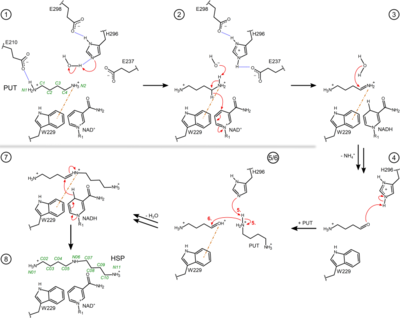
| + | ''Bp''BADH has a prototypical BADH topology and shares considerable structure and sequence similarity with the ortholog from ''P. aeruginosa'' (''Pa''BADH); see static image below: | ||
| + | [[Image:Figure3pahss3.png|left|400px|thumb|Figure 3. Proposed reaction steps of the conversion of PUT to HSP by the bacterial HSS. Relevant residues, NAD(H), PUT, HSP and intermediates are shown as two-dimensional structure representations. Hydrogen bonds are depicted as blue dotted lines, delocalized electrons as dashed lines, cation-π interactions as orange dash-dotted lines and electron transfers as red arrows. Atom numbering is given for PUT and HSP in green. For simplicity, steps 5 and 6 are shown in combined depictions with correspondingly labeled electron transfers. A more detailed sequence of reaction steps was described before<ref name="Krossa">PMID:26776105</ref> and additional intervening reaction steps are proposed in Fig. S2 of Helfrich & Scheidig, 2021<ref name="Helfrich1">PMID:34605434</ref>.]] | ||
| + | {{Clear}} | ||
| + | |||
The structures are similar to those of BADH from ''Pseudomonas aeruginosa'' (''Pa''BADH). The co-factor binding domains of ''Bp''BADH ([[6wsb]]) and ''Pa''BADH ([[4caz]]) are well conserved (identical residues of both structures are labeled in green, while non-identical in red): | The structures are similar to those of BADH from ''Pseudomonas aeruginosa'' (''Pa''BADH). The co-factor binding domains of ''Bp''BADH ([[6wsb]]) and ''Pa''BADH ([[4caz]]) are well conserved (identical residues of both structures are labeled in green, while non-identical in red): | ||
*<scene name='89/899476/Cv/11'>Co-factor binding domain of BpBADH</scene> ([[6wsb]]). | *<scene name='89/899476/Cv/11'>Co-factor binding domain of BpBADH</scene> ([[6wsb]]). | ||
Revision as of 16:35, 20 December 2021
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.