Inositol 1,4,5-Trisphosphate Receptor
From Proteopedia
| (36 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | < | + | <StructureSection load='1n4K' size='350' side='right' caption='Mouse inositol triphosphate receptor ligand-binding core complex with its ligand inositol triphosphate (PDB entry [[1n4k]])' scene='38/382942/Cv/1'> |
| - | + | ==Introduction== | |
| - | + | [[Inositol 1,4,5-Trisphosphate Receptor]] binding protein is a ubiquitous protein involved in the Ca<sup>2+</sup> signalling processes in a variety of organisms <ref name="mainpaper">PMID:12442173</ref>. See also [[Endoplasmic reticulum/Sarcoplasmic reticulum receptors]], [[Receptor]] and [[Ca2+ signalling processes]]. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | Inositol 1,4,5- | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | [[ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ==Structure== | ||
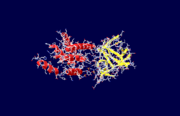
| + | The specific type of inositol 1,4,5-trisphosphate receptor (InsP<sub>3</sub>R) protein discussed here is the mouse type 1 InsP<sub>3</sub>R, also called InsP<sub>3</sub>R1. This polypeptide contains three major regions: the <scene name='38/382942/N_terminal_domain/1'>amino terminal</scene> inositol 1,4,5-trisphosphate (InsP<sub>3</sub>) binding region, the central modulatory region, and the <scene name='38/382942/C_terminal_domain/1'>carboxy-terminus channel region</scene>.<ref name="mainpaper"/> The protein forms an L-shaped structure composed of two asymmetric domains perpendicular to each other.<ref name="mainpaper"/> The N-terminal domain is made up of 12 β-strands and 2 single-turn helices, which come together to form a barrel.<ref name="mainpaper"/> The C-terminal end is quite different, consisting of a bundle made of eight α-helices.<ref name="mainpaper"/> The interface of the two domains is lined with basic residues and forms the <scene name='38/382942/Ip3_binding_pocket/1'>receptor site</scene> for InsP<sub>3</sub>.<ref name="mainpaper"/> The InsP<sub>3</sub>R protein does not belong to a superfamily of proteins. The receptor is thought to span the membrane 6 times, leaving the C-terminus in the cytoplasm.<ref name="functionref"/> | ||
=== Domain Structure === | === Domain Structure === | ||
| - | |||
The protein fold of the β-domain can also be called the β-trefoil. This element is present in other proteins as well, including fibroblast growth factors and mannose receptors.<ref name="mainpaper"/> In the case of the InsP<sub>3</sub>R β-trefoil, the structure was found to be very similar to the β-trefoil of the mannose receptor.<ref name="mainpaper"/> In the β-domain of InsP<sub>3</sub>R1, three of six two-stranded hairpins come together to form a barrel and the other three form a triangular cap for the barrel.<ref name="mainpaper"/> | The protein fold of the β-domain can also be called the β-trefoil. This element is present in other proteins as well, including fibroblast growth factors and mannose receptors.<ref name="mainpaper"/> In the case of the InsP<sub>3</sub>R β-trefoil, the structure was found to be very similar to the β-trefoil of the mannose receptor.<ref name="mainpaper"/> In the β-domain of InsP<sub>3</sub>R1, three of six two-stranded hairpins come together to form a barrel and the other three form a triangular cap for the barrel.<ref name="mainpaper"/> | ||
| Line 26: | Line 12: | ||
The α-domain of InsP<sub>3</sub>R shows a high degree of homology with an element called an armidillo repeat fold found in proteins such as β-catenin and importins.<ref name="mainpaper"/> In β-catenin and importins, the armadillo repeat functions as a motif for protein-protein interactions.<ref name="mainpaper"/> Within the α-domain of mouse InsP<sub>3</sub>R1, there are two large, highly conserved surfaces.<ref name="mainpaper"/> Both regions are rich in aromatic residues, indicating that they may function as interaction sites for parts of the receptor or other cellular proteins.<ref name="mainpaper"/> A possible option for this kind of binding domain would be the InsP<sub>3</sub> binding suppressor domain present at the N-terminus which reduces the binding affinity for the InsP<sub>3</sub> ligand.<ref name="mainpaper"/> | The α-domain of InsP<sub>3</sub>R shows a high degree of homology with an element called an armidillo repeat fold found in proteins such as β-catenin and importins.<ref name="mainpaper"/> In β-catenin and importins, the armadillo repeat functions as a motif for protein-protein interactions.<ref name="mainpaper"/> Within the α-domain of mouse InsP<sub>3</sub>R1, there are two large, highly conserved surfaces.<ref name="mainpaper"/> Both regions are rich in aromatic residues, indicating that they may function as interaction sites for parts of the receptor or other cellular proteins.<ref name="mainpaper"/> A possible option for this kind of binding domain would be the InsP<sub>3</sub> binding suppressor domain present at the N-terminus which reduces the binding affinity for the InsP<sub>3</sub> ligand.<ref name="mainpaper"/> | ||
| - | + | [[Image:1n4k2.png|thumb|The two domains of the inositol 1,4,5-trisphosphate receptor protein. The yellow ribbons represent the β-domain and the red helices represent the α-domain]] | |
| - | [[Image: | + | |
| - | + | ||
| - | + | ||
| - | The | + | |
| - | + | ||
| - | + | ||
=== Binding the InsP<sub>3</sub> Ligand: Mechanism and Structural Components=== | === Binding the InsP<sub>3</sub> Ligand: Mechanism and Structural Components=== | ||
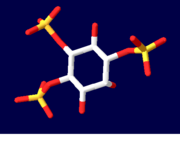
| - | The | + | The InsP<sub>3</sub> <scene name='Sandbox_170/1n4k/8'>ligand</scene> sits between the two domains of the protein. Highly <scene name='38/382942/Ip3_binding_pocket/1'>basic amino acid residues</scene> are present on both domains and are responsible for the binding of InsP<sub>3</sub> to InsP<sub>3</sub>R.<ref name="mainpaper"/> Since the InsP<sub>3</sub> ligand is highly charged, it is very likely to interact with the positively charged amino acids present in the N-terminus InsP<sub>3</sub>-binding domain.<ref name="functionref"/> In binding, water molecules are involved in hydrogen bonding between InsP<sub>3</sub> and its receptor as well as interactions between protein side chains and phosphorous.<ref name="mainpaper"/> <scene name='38/382942/Cv/4'>Active site</scene> (water molecules shown as red spheres). Coordination of phosphorous groups is mediated by residues in both the β-domain and α-domain. The hydroxyl groups of InsP<sub>3</sub> play a small role in binding to InsP<sub>3</sub>.<ref name="mainpaper"/> Additionally, 9 out of 12 Arg/Lys residues play a very important role in ligand binding and salt bridges to stabilize between the domain regions.<ref name="mainpaper"/> The non-basic residues T266, T267, G268, and Y567 are also integral in InsP<sub>3</sub> coordination: if T267, G268 or Y567 residues are mutated then there will be a significant reduction in ligand binding.<ref name="mainpaper"/> In all likelihood, the InsP<sub>3</sub>-binding site has been found to be made up of multiple sequences present throughout the N-terminal area of the protein.<ref name="functionref"/> This makes the tertiary structure of the protein and proper folding absolutely integral to the function: if the protein does not fold correctly, then the multiple sequences of the protein making up the binding region cannot come together to be at all functional in binding the InsP<sub>3</sub> ligand. |
| - | + | ||
[[Image:Ligand1.PNG| thumb|Inositol 1,4,5-trisphosphate]] | [[Image:Ligand1.PNG| thumb|Inositol 1,4,5-trisphosphate]] | ||
| - | |||
| - | |||
| - | |||
== Function == | == Function == | ||
| Line 56: | Line 32: | ||
A very important property of the receptor is that it is regulated by Ca<sup>2+</sup> concentrations. Lower concentrations make the receptor more sensitive to InsP<sub>3</sub> while high concentrations can inhibit the receptor activity.<ref name="functionref"/> Also, the receptor itself can bind Ca<sup>3</sup> itself at more than one site. A Ca<sup>2+</sup> binding site within the ligand binding domain may even suggest that these Ca<sup>2+</sup> binding sites are involved in the effects Ca<sup>2+</sup> has on InsP<sub>3</sub> binding to its ligand. | A very important property of the receptor is that it is regulated by Ca<sup>2+</sup> concentrations. Lower concentrations make the receptor more sensitive to InsP<sub>3</sub> while high concentrations can inhibit the receptor activity.<ref name="functionref"/> Also, the receptor itself can bind Ca<sup>3</sup> itself at more than one site. A Ca<sup>2+</sup> binding site within the ligand binding domain may even suggest that these Ca<sup>2+</sup> binding sites are involved in the effects Ca<sup>2+</sup> has on InsP<sub>3</sub> binding to its ligand. | ||
| - | The method of regulation by ATP on the receptor is very similar to that of Ca<sup> | + | The method of regulation by ATP on the receptor is very similar to that of Ca<sup>2+</sup>. Increased ATP concentrations increase receptor activity whereas higher concentrations decrease receptor activity.<ref name="functionref"/> The stimulatory activity of ATP likely occurs through consensus adenine nucleotide-binding motifs.<ref name="functionref"/> The inhibitory effect of ATP is thought to arise through its charged nature, acting as a competitive antagonist at the InsP<sub>3</sub>-binding site.<ref name="functionref"/> |
The InsP<sub>3</sub>R protein can autophosphorylate itself and is a substrate for multiple protein kinases.<ref name="functionref"/> These kinases include cyclic AMP-dependent protein kinase (PKA), cyclic GMP-dependent protein kinase (PKG) and others.<ref name="functionref"/> The protein kinases are thought to interact with the InsP<sub>3</sub> receptor by controlling the sensitivity to Ca<sup>2+</sup> in different tissues as well as affecting the sensitivity of InsP<sub>3</sub> itself to Ca<sup>2+</sup>.<ref name="functionref"/> | The InsP<sub>3</sub>R protein can autophosphorylate itself and is a substrate for multiple protein kinases.<ref name="functionref"/> These kinases include cyclic AMP-dependent protein kinase (PKA), cyclic GMP-dependent protein kinase (PKG) and others.<ref name="functionref"/> The protein kinases are thought to interact with the InsP<sub>3</sub> receptor by controlling the sensitivity to Ca<sup>2+</sup> in different tissues as well as affecting the sensitivity of InsP<sub>3</sub> itself to Ca<sup>2+</sup>.<ref name="functionref"/> | ||
| + | </StructureSection> | ||
| + | ===3D structures of inositol 1,4,5-trisphosphate receptor=== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | + | [[6dqj]], [[6dr2]], [[6dra]], [[6uqk]] – hInsP3R III – human - Cryo EM<br /> | |
| + | [[6dqn]], [[6dqs]], [[6dqv]], [[6dqz]], [[6dr0]], [[6drc]] – hInsP3R III + IP3 - Cryo EM<br /> | ||
| + | [[5x9z]], [[5xa0]] – mInsP3R I - mouse<br /> | ||
| + | [[3jrr]] – mInsP3R III ligand-binding suppressor domain <br /> | ||
| + | [[1xzz]] - mInsP3R I ligand-binding suppressor domain<br /> | ||
| + | [[1n4k]] - mInsP3R I receptor-binding core + IP3<br /> | ||
| + | [[5gug]], [[5xa1]] – mInsP3R I + IP3<br /> | ||
| + | [[3t8s]], [[3uj4]] - rInsP3R I ligand-binding domain - rat<br /> | ||
| + | [[3uj0]] - rInsP3R I ligand-binding domain + IP3<br /> | ||
| + | [[3jav]], [[6mu2]] – rInsP3R I – Cryo EM<br /> | ||
| + | [[7lhe]], [[7lhf]] – rInsP3R I + lipid – Cryo EM<br /> | ||
| + | [[6mu1]] – rInsP3R I + adenophostin – Cryo EM<br /> | ||
==References== | ==References== | ||
| - | <references /> | + | <references /> |
| - | + | [[Category:Topic Page]] | |
Current revision
| |||||||||||
3D structures of inositol 1,4,5-trisphosphate receptor
Updated on 19-January-2022
6dqj, 6dr2, 6dra, 6uqk – hInsP3R III – human - Cryo EM
6dqn, 6dqs, 6dqv, 6dqz, 6dr0, 6drc – hInsP3R III + IP3 - Cryo EM
5x9z, 5xa0 – mInsP3R I - mouse
3jrr – mInsP3R III ligand-binding suppressor domain
1xzz - mInsP3R I ligand-binding suppressor domain
1n4k - mInsP3R I receptor-binding core + IP3
5gug, 5xa1 – mInsP3R I + IP3
3t8s, 3uj4 - rInsP3R I ligand-binding domain - rat
3uj0 - rInsP3R I ligand-binding domain + IP3
3jav, 6mu2 – rInsP3R I – Cryo EM
7lhe, 7lhf – rInsP3R I + lipid – Cryo EM
6mu1 – rInsP3R I + adenophostin – Cryo EM
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 Bosanac I, Alattia JR, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, Mikoshiba K, Ikura M. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002 Dec 12;420(6916):696-700. Epub 2002 Nov 17. PMID:12442173 doi:10.1038/nature01268
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 Patel S, Joseph SK, Thomas AP. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium. 1999 Mar;25(3):247-64. PMID:10378086 doi:10.1054/ceca.1999.0021
Proteopedia Page Contributors and Editors (what is this?)
Shannon King, Alexander Berchansky, Michal Harel, Ann Taylor, David Canner, Andrea Gorrell, Jaclyn Gordon