Methionine adenosyltransferase
From Proteopedia
| Line 16: | Line 16: | ||
Mammalian MAT consists of α and β subunits. The MATα1 and MATα2 subunits are catalytic subunits while MATβ is a regulatory subunit. Human MATα2 subunits can form <scene name='49/493038/Tetramer/1'>tetramers</scene>, where the active site is found <scene name='49/493038/Tetramer/2'>between two of the subunits</scene>. (For comparison, here is the <scene name='49/493038/Cv/13'>overall structure</scene> and the <scene name='49/493038/Cv/12'>active site</scene> of the rat enzyme.<ref>PMID:12888348</ref>) | Mammalian MAT consists of α and β subunits. The MATα1 and MATα2 subunits are catalytic subunits while MATβ is a regulatory subunit. Human MATα2 subunits can form <scene name='49/493038/Tetramer/1'>tetramers</scene>, where the active site is found <scene name='49/493038/Tetramer/2'>between two of the subunits</scene>. (For comparison, here is the <scene name='49/493038/Cv/13'>overall structure</scene> and the <scene name='49/493038/Cv/12'>active site</scene> of the rat enzyme.<ref>PMID:12888348</ref>) | ||
| - | The <scene name='49/493038/ | + | The <scene name='49/493038/Tetramer/4'>substrates</scene> used by the enzyme are methionine and ATP. Notably, ATP is not used as a source of energy in this reaction like it is for many other processes. Instead, it is used as a substrate in the synthesis reaction. Methionine and ATP enter the active site and are stabilized by residues present there, including lysine and histidine. Once the reaction begins to take place, methionine flips toward the 5' carbon of the adenosine sugar<ref>doi:10.1042/BJ20121580</ref>. Following nucleophilic attack of the sulfur on the carbon, the C-O bond between the phosphates and the carbon breaks, and the <scene name='49/493038/Product/5'>products</scene> are formed (tripolyphosphate not pictured). SAM is released from the active site first. MAT also catalyzes hydrolysis of the tripolyphosphate into pyrophosphate and orthophosphate, which are then released from the active site <ref>Niland CN, Ghosh A, Cahill SM, Schramm VL. Mechanism and Inhibition of Human Methionine Adenosyltransferase 2A. ACS Biochemistry. 2021 Mar 3;60 (10) 791-801. doi: https://doi.org/10.1021/acs.biochem.0c00998</ref>. |
The subunits are encoded on different genes in humans, so they are created separately and can then come together to form various complexes, such as MATαβ or MATα2 dimers <ref name="Murray et al." />. Not much is currently known about the function of this regulatory subunit and how it regulates the function of the enzyme<ref>DOI:10.1107/S2052252514012585</ref>. However, Murray et al.<ref name="Murray et al." /> show that even in the absence of the regulatory subunit, the active site found in the catalytic subunit remains functional. | The subunits are encoded on different genes in humans, so they are created separately and can then come together to form various complexes, such as MATαβ or MATα2 dimers <ref name="Murray et al." />. Not much is currently known about the function of this regulatory subunit and how it regulates the function of the enzyme<ref>DOI:10.1107/S2052252514012585</ref>. However, Murray et al.<ref name="Murray et al." /> show that even in the absence of the regulatory subunit, the active site found in the catalytic subunit remains functional. | ||
Revision as of 21:33, 25 April 2022
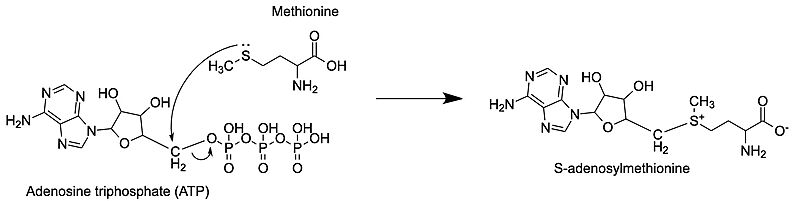
Methionine adenosyltransferase (MAT) or S-adenosylmethionine synthetase (SAM synthetase) synthesizes S-adenosylmethionine (SAM or AdoMet) from the substrates adenosine triphosphate (ATP) and methionine. ATP isn’t used only as a source of energy like it is in other reactions but gets a methionine added onto the 5' carbon while the three phosphate groups are broken down and released from the active site. This enzyme is conserved and found in many organisms, so it is essential for life. Importantly, the production of SAM by MAT provides methyl for methylation of nucleic acids, allowing for epigenetic modification. Problems with this enzyme have been shown to cause diseases including various cancers.
Contents |
Relevance
The product of this enzymatic reaction, SAM, is the universal methyl donor of metabolism. DNA methyltransferases can transfer a methyl group from SAM to the 5th carbon of cytosine residues [1]. In this way, MAT is indirectly important for regulation of gene expression by providing methyl through SAM. SAM is also involved in N-methylation, O-methylation and C-methylation, yielding S-adenosyl homocysteine as a product that gets recycled by the one-carbon metabolism. Radical SAM enzymes break down SAM into an adenosyl radical and methionine, enabling a host of otherwise difficult to achieve reactions, e.g. in molybdenum cofactors biosynthesis[2]. Accumulation of S-adenosyl homocysteine (or homocysteine itself) indicates an imbalance in supply and demand for SAM in the organism. Methionine metabolism impairment in liver diseases is related in alteration in MAT[3].
Function and reaction mechanism
S-adenosylmethionine synthetase or S-adenosylmethionine synthase or S-adenosylmethionine transferase or methionine adenosyltransferase (MAT) catalyzes the conversion of methionine and ATP to S-adenosylmethionine (AdoMet), pyrophosphate (PPi) and orthophosphate (Pi). The catalytic entity of MAT is a dimer. MAT cofactors are Mg+2 (or Co+2) and K+ ions[4].
The nucleophilic sulfur atom of methionine attacks the slightly positive 5' carbon of the adenosine sugar unit. Following this, the bond from the 5' carbon to the oxygen breaks, separating the tripolyphosphate from the newly formed S-adenosylmethionine (SAM) [5]. This is an example of an SN2 reaction, where the substrates move through a transition state to then form the products. The products are only released after the methionine binds and the C-O bond breaks.
Structure
| |||||||||||
3D structures of S-adenosylmethionine synthetase
S-adenosylmethionine synthetase 3D structures
25-April-2022
References
- ↑ Moore LD, Le T, Fan G. DNA methylation and its basic function. Neuropsychopharmacology. 2013 Jan;38(1):23-38. doi: 10.1038/npp.2012.112. Epub, 2012 Jul 11. PMID:22781841 doi:http://dx.doi.org/10.1038/npp.2012.112
- ↑ Hanzelmann P, Schindelin H. Crystal structure of the S-adenosylmethionine-dependent enzyme MoaA and its implications for molybdenum cofactor deficiency in humans. Proc Natl Acad Sci U S A. 2004 Aug 31;101(35):12870-5. Epub 2004 Aug 18. PMID:15317939 doi:10.1073/pnas.0404624101
- ↑ Mato JM, Alvarez L, Ortiz P, Mingorance J, Duran C, Pajares MA. S-adenosyl-L-methionine synthetase and methionine metabolism deficiencies in cirrhosis. Adv Exp Med Biol. 1994;368:113-7. PMID:7741002
- ↑ Takusagawa F, Kamitori S, Markham GD. Structure and function of S-adenosylmethionine synthetase: crystal structures of S-adenosylmethionine synthetase with ADP, BrADP, and PPi at 28 angstroms resolution. Biochemistry. 1996 Feb 27;35(8):2586-96. PMID:8611562 doi:http://dx.doi.org/10.1021/bi952604z
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Murray B, Antonyuk SV, Marina A, Lu SC, Mato JM, Hasnain SS, Rojas Al. Crystallography captures catalytic steps in human methionine adenosyltransferase enzymes. PNAS. 2016 Feb 8;113 (8) 2104-2109. doi: https://doi.org/10.1073/pnas.1510959113
- ↑ Gonzalez B, Pajares MA, Hermoso JA, Guillerm D, Guillerm G, Sanz-Aparicio J. Crystal structures of methionine adenosyltransferase complexed with substrates and products reveal the methionine-ATP recognition and give insights into the catalytic mechanism. J Mol Biol. 2003 Aug 8;331(2):407-16. PMID:12888348
- ↑ Shafqat N, Muniz JR, Pilka ES, Papagrigoriou E, von Delft F, Oppermann U, Yue WW. Insight into S-adenosylmethionine biosynthesis from the crystal structures of the human methionine adenosyltransferase catalytic and regulatory subunits. Biochem J. 2013 May 15;452(1):27-36. doi: 10.1042/BJ20121580. PMID:23425511 doi:10.1042/BJ20121580
- ↑ Niland CN, Ghosh A, Cahill SM, Schramm VL. Mechanism and Inhibition of Human Methionine Adenosyltransferase 2A. ACS Biochemistry. 2021 Mar 3;60 (10) 791-801. doi: https://doi.org/10.1021/acs.biochem.0c00998
- ↑ Murray B, Antonyuk SV, Marina A, Van Liempd SM, Lu SC, Mato JM, Hasnain SS, Rojas AL. Structure and function study of the complex that synthesizes S-adenosylmethionine. IUCrJ. 2014 Jun 12;1(Pt 4):240-9. doi: 10.1107/S2052252514012585. eCollection, 2014 Jul 1. PMID:25075345 doi:http://dx.doi.org/10.1107/S2052252514012585
Proteopedia Page Contributors and Editors (what is this?)
Karsten Theis, Anna Postnikova, Michal Harel, Kia Yang, Michael O'Shaughnessy, Alexander Berchansky, Jaime Prilusky