Journal:IUCrJ:S2052252521008125
From Proteopedia
(Difference between revisions)

| (8 intermediate revisions not shown.) | |||
| Line 11: | Line 11: | ||
{{Clear}} | {{Clear}} | ||
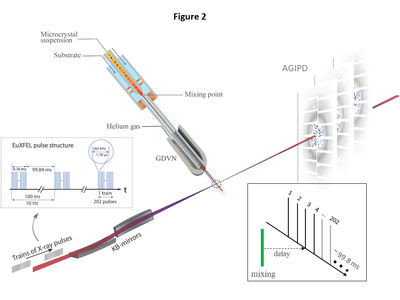
| - | We follow the increase of the CEF occupancy in the catalytic cleft of BlaC from 5 ms to 50 ms after mixing with CEF by XFEL based MISC experiments. | + | We follow the increase of the CEF occupancy in the catalytic cleft of BlaC from 5 ms to 50 ms after mixing with CEF by XFEL based MISC experiments. At the edge of the crystal, almost all active BlaC subunits (B and D) are bound to CEF already at 5 ms. In the center the BlaC-CEF complex concentration is 0.21 mmol/L (2.7 % of the total concentration of B and D subunits in the crystal), although the CEF concentration delivered by diffusion is already 35 mmol/L (which is 23 % of the outside CEF concentration but 5.5 times the stoichiometric concentration). The situation changes completely at 30 ms, where almost 100 % occupancy is reached everywhere in the crystal which is in accordance with earlier results (Olmos ''et al.,'' 2018)<ref>PMID:29848358</ref>, and with the occupancy at Δtm = 50 ms reported here (see movie below) |
| + | |||
| + | <qt>file=Image:Supplementary_movie_2a.mp4|autoplay=false|width=400|height=400|controller=true|loop=false</qt><br/> | ||
| + | {{Clear}} | ||
| + | |||
| + | Steps of the CEF-BlaC binding: | ||
*<scene name='88/889829/Cv/8'>0 ms, the CEF ligand is not yet diffused in, the phosphate (Pi) from the crystallization buffer is dominant in the active site</scene>. | *<scene name='88/889829/Cv/8'>0 ms, the CEF ligand is not yet diffused in, the phosphate (Pi) from the crystallization buffer is dominant in the active site</scene>. | ||
*<scene name='88/889829/Cv/7'>5 ms, the phosphate is beginning to be displaced by CEF</scene>. | *<scene name='88/889829/Cv/7'>5 ms, the phosphate is beginning to be displaced by CEF</scene>. | ||
| Line 18: | Line 23: | ||
remains</scene>. | remains</scene>. | ||
| - | See also the movie below: | + | See also the movie below, which visualizes how the CEF interacts with the BlaC: |
| - | <qt>file=Image: | + | <qt>file=Image:Supplementary_movie_Polder1.mp4|autoplay=false|width=400|height=400|controller=true|loop=false</qt><br/> |
{{Clear}} | {{Clear}} | ||
| Line 27: | Line 32: | ||
In addition to the CEF binding we observe the reaction of <scene name='88/889829/Cv/14'>BlaC with an inhibitor sulbactam</scene> (SUB) at 66 ms. SUB reacts to a so-called <scene name='88/889829/Cv/15'>trans-enamine in subunits B and D of the BlaC</scene> already after 66 ms. However, <scene name='88/889829/Cv/14'>SUB stays intact in subunits A and C</scene>. As the reaction proceeds to the ''trans''-enamine also in A and C, the structures of the weakly bound SUBs in these subunits at 66 ms represent an interesting intermediate that would not have been detected in static structures. | In addition to the CEF binding we observe the reaction of <scene name='88/889829/Cv/14'>BlaC with an inhibitor sulbactam</scene> (SUB) at 66 ms. SUB reacts to a so-called <scene name='88/889829/Cv/15'>trans-enamine in subunits B and D of the BlaC</scene> already after 66 ms. However, <scene name='88/889829/Cv/14'>SUB stays intact in subunits A and C</scene>. As the reaction proceeds to the ''trans''-enamine also in A and C, the structures of the weakly bound SUBs in these subunits at 66 ms represent an interesting intermediate that would not have been detected in static structures. | ||
{{Clear}} | {{Clear}} | ||
| + | |||
| + | '''PDB references:''' BlaC, unmixed, [[7k8l]]; mixed with ceftriaxone, 5 ms, [[7k8e]]; 10 ms, [[7k8f]]; 50 ms, [[7k8h]]; mixed with sulbactam, 66 ms, [[7k8k]]. | ||
<b>References</b><br> | <b>References</b><br> | ||
Current revision
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.