Journal:Acta Cryst F:S2053230X20011310
From Proteopedia
(Difference between revisions)

| (20 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='' size='450' side='right' scene='85/859036/Cv/ | + | <StructureSection load='' size='450' side='right' scene='85/859036/Cv/14' caption=''> |
===Crystallization and structure of ebselen bound to cysteine 141 of human inositol monophosphatase (IMPase).=== | ===Crystallization and structure of ebselen bound to cysteine 141 of human inositol monophosphatase (IMPase).=== | ||
<big>Gareth D. Fenn, Helen Waller-Evans, John R. Atack and Benjamin D. Bax</big> <ref>doi 10.1107/S2053230X20011310</ref> | <big>Gareth D. Fenn, Helen Waller-Evans, John R. Atack and Benjamin D. Bax</big> <ref>doi 10.1107/S2053230X20011310</ref> | ||
| Line 5: | Line 5: | ||
<b>Molecular Tour</b><br> | <b>Molecular Tour</b><br> | ||
Inositol monophosphatase (IMPase) is inhibited by lithium, the most efficacious treatment for bipolar disorder. Several therapies have been approved, or are going through clinical trials, aimed at the replacement of lithium in the treatment of bipolar disorder. One candidate small molecule is ebselen, a selenium-containing antioxidant, which has been demonstrated to produce lithium-like effects, both in a murine model and in clinical trials. | Inositol monophosphatase (IMPase) is inhibited by lithium, the most efficacious treatment for bipolar disorder. Several therapies have been approved, or are going through clinical trials, aimed at the replacement of lithium in the treatment of bipolar disorder. One candidate small molecule is ebselen, a selenium-containing antioxidant, which has been demonstrated to produce lithium-like effects, both in a murine model and in clinical trials. | ||
| + | |||
| + | View of ebselen attached to Cys141 (PDB entry [[6zk0]]): | ||
| + | *<scene name='85/859036/Cv/17'>Overview of two ebselen molecules attached to A and A' (symmetry related) subunits around crystallographic twofold axis</scene>. One subunit (A) has carbons in orange and the second (A') subunit has carbons slate-blue (nitrogens are blue, carbons red, seleniums orange and sulphurs gold). Water molecules are shown as red sphers. | ||
| + | |||
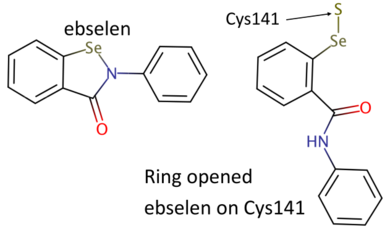
| + | [[Image:Ebs1.png|thumb|390px|left|Chemical structures of ebselen and ring-open ebselen on Cys141 (drawn with Marvin, [https://www.chemaxon.com https://www.chemaxon.com]).]] | ||
| + | {{Clear}} | ||
| + | |||
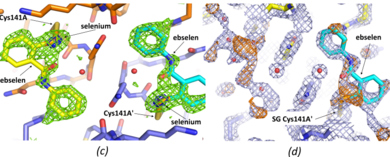
| + | [[Image:Ebs4.png|thumb|390px|left|(c) Final ebselen omit map (Fo-Fc) (3 sigma green, 15 sigma blue). Note peaks on seleniums (blue mesh) are 20.5 and 19.6 sigma in this ebselen omit map. (d) Original DIMPLE (Wojdyr ''et al.,'' 2013<ref name="Wojdyr">Wojdyr, M., Keegan, R., Winter, G. & Ashton, A. (2013). Acta Cryst. A69, s299</ref>) 2Fo-Fc map (1sigma – light blue), and difference map Fo-Fc (3 sigma - orange). For subunit A the DIMPLE refined structure with waters (small red spheres) refined into the density for the ebselen is shown. For the A' subunit the 'final' coordinates (including ebselen) are shown.]] | ||
| + | {{Clear}} | ||
Here we present the crystallization and first structure of human IMPase covalently complexed with ebselen, a 1.47 Å crystal structure (PDB entry [[6zk0]]). In the human-IMPase-complex ebselen, in a ring opened conformation, is covalently attached to Cys141, a residue located away from the active site. | Here we present the crystallization and first structure of human IMPase covalently complexed with ebselen, a 1.47 Å crystal structure (PDB entry [[6zk0]]). In the human-IMPase-complex ebselen, in a ring opened conformation, is covalently attached to Cys141, a residue located away from the active site. | ||
| - | IMPase is a dimeric enzyme and, in the crystal structure, two adjacent dimers share four ebselen molecules, creating a tetramer with ~222 symmetry. In the crystal structure presented in this publication, the active site in the tetramer is still accessible, suggesting that ebselen may function as an allosteric inhibitor, or may block the binding of partner proteins. | + | IMPase is a dimeric enzyme and, in the crystal structure, two adjacent dimers share four ebselen molecules, creating a tetramer with ~222 symmetry. In the crystal structure presented in this publication, the active site in the tetramer is still accessible, suggesting that ebselen may function as an allosteric inhibitor, or may block the binding of partner proteins. |
Orthogonal views of IMPase dimer showing ebselen on Cys141 and metal ions in the active sites based on the structure of PDB entry [[6zk0]]: | Orthogonal views of IMPase dimer showing ebselen on Cys141 and metal ions in the active sites based on the structure of PDB entry [[6zk0]]: | ||
| Line 13: | Line 22: | ||
*<scene name='85/859036/Cv/5'>Orthogonal view</scene>. | *<scene name='85/859036/Cv/5'>Orthogonal view</scene>. | ||
*<scene name='85/859036/Cv/6'>Orthogonal view</scene>. | *<scene name='85/859036/Cv/6'>Orthogonal view</scene>. | ||
| + | |||
Orthogonal views of IMPase tetramer showing ebselen on Cys141 and metal ions in the active sites based on the structure of PDB entry [[6zk0]]: | Orthogonal views of IMPase tetramer showing ebselen on Cys141 and metal ions in the active sites based on the structure of PDB entry [[6zk0]]: | ||
*<scene name='85/859036/Cv/7'>Tetramer, 1st orientation</scene>. The two subunits in the dimer are shown as green (subunit A) and cyan (subunit B) cartoons with ebselen attached to Cys141A and Cys141B in space-fill. Subunit A' is in yellow and B' in magenta. Metal ions (Mn2+/Na+) at each active site are shown as spheres. | *<scene name='85/859036/Cv/7'>Tetramer, 1st orientation</scene>. The two subunits in the dimer are shown as green (subunit A) and cyan (subunit B) cartoons with ebselen attached to Cys141A and Cys141B in space-fill. Subunit A' is in yellow and B' in magenta. Metal ions (Mn2+/Na+) at each active site are shown as spheres. | ||
| + | *<scene name='85/859036/Cv/8'>Orthogonal view</scene>. This view is along the crystallographic twofold that rotates the A-B dimer (green/cyan) onto the A'-B' dimer (yellow-magenta). | ||
| + | *<scene name='85/859036/Cv/9'>Orthogonal view</scene>. | ||
| + | |||
| + | Two views of the tetramer from 'underneath'. Showing that the three metal ions (spheres) at each active site are still accessible in the tetramer: | ||
| + | *<scene name='85/859036/Cv/10'>The tetramer from 'underneath' view</scene>. | ||
| + | *<scene name='85/859036/Cv/11'>The tetramer from 'underneath' view</scene>. In this view both dimers are shown in space-fill. | ||
| + | |||
| + | '''PDB reference:''' human IMPase bound to ebselen, [[6zk0]] | ||
<b>References</b><br> | <b>References</b><br> | ||
Current revision
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.