Journal:Acta Cryst F:S2053230X20011310
From Proteopedia
(Difference between revisions)

| (14 intermediate revisions not shown.) | |||
| Line 7: | Line 7: | ||
View of ebselen attached to Cys141 (PDB entry [[6zk0]]): | View of ebselen attached to Cys141 (PDB entry [[6zk0]]): | ||
| - | *<scene name='85/859036/Cv/17'>Overview of two ebselen molecules attached to A and A' (symmetry related) subunits around crystallographic twofold axis</scene>. One subunit (A) has carbons in orange and the second (A') subunit has carbons slate-blue (nitrogens are blue, carbons red, seleniums orange and sulphurs gold). | + | *<scene name='85/859036/Cv/17'>Overview of two ebselen molecules attached to A and A' (symmetry related) subunits around crystallographic twofold axis</scene>. One subunit (A) has carbons in orange and the second (A') subunit has carbons slate-blue (nitrogens are blue, carbons red, seleniums orange and sulphurs gold). Water molecules are shown as red sphers. |
| + | |||
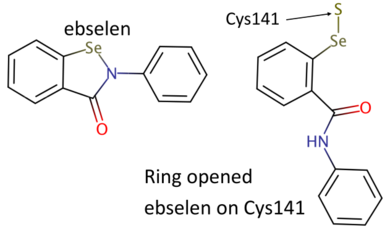
| + | [[Image:Ebs1.png|thumb|390px|left|Chemical structures of ebselen and ring-open ebselen on Cys141 (drawn with Marvin, [https://www.chemaxon.com https://www.chemaxon.com]).]] | ||
| + | {{Clear}} | ||
| + | |||
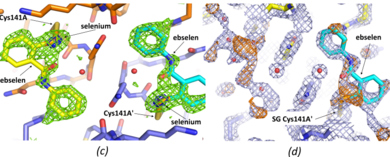
| + | [[Image:Ebs4.png|thumb|390px|left|(c) Final ebselen omit map (Fo-Fc) (3 sigma green, 15 sigma blue). Note peaks on seleniums (blue mesh) are 20.5 and 19.6 sigma in this ebselen omit map. (d) Original DIMPLE (Wojdyr ''et al.,'' 2013<ref name="Wojdyr">Wojdyr, M., Keegan, R., Winter, G. & Ashton, A. (2013). Acta Cryst. A69, s299</ref>) 2Fo-Fc map (1sigma – light blue), and difference map Fo-Fc (3 sigma - orange). For subunit A the DIMPLE refined structure with waters (small red spheres) refined into the density for the ebselen is shown. For the A' subunit the 'final' coordinates (including ebselen) are shown.]] | ||
| + | {{Clear}} | ||
Here we present the crystallization and first structure of human IMPase covalently complexed with ebselen, a 1.47 Å crystal structure (PDB entry [[6zk0]]). In the human-IMPase-complex ebselen, in a ring opened conformation, is covalently attached to Cys141, a residue located away from the active site. | Here we present the crystallization and first structure of human IMPase covalently complexed with ebselen, a 1.47 Å crystal structure (PDB entry [[6zk0]]). In the human-IMPase-complex ebselen, in a ring opened conformation, is covalently attached to Cys141, a residue located away from the active site. | ||
| Line 25: | Line 31: | ||
*<scene name='85/859036/Cv/10'>The tetramer from 'underneath' view</scene>. | *<scene name='85/859036/Cv/10'>The tetramer from 'underneath' view</scene>. | ||
*<scene name='85/859036/Cv/11'>The tetramer from 'underneath' view</scene>. In this view both dimers are shown in space-fill. | *<scene name='85/859036/Cv/11'>The tetramer from 'underneath' view</scene>. In this view both dimers are shown in space-fill. | ||
| + | |||
| + | '''PDB reference:''' human IMPase bound to ebselen, [[6zk0]] | ||
<b>References</b><br> | <b>References</b><br> | ||
Current revision
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.