Journal:Acta Cryst F:S2053230X21008967
From Proteopedia
(Difference between revisions)

| (11 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='' size='450' side='right' scene=' | + | <StructureSection load='' size='450' side='right' scene='89/891404/Cv/1' caption=''> |
===Microcrystal preparation for serial femtosecond X-ray crystallography of bacterial copper amine oxidase=== | ===Microcrystal preparation for serial femtosecond X-ray crystallography of bacterial copper amine oxidase=== | ||
<big>Takeshi Murakawa, Mamoru Suzuki, Toshi Arima, Michihiro Sugahara, Tomoyuki | <big>Takeshi Murakawa, Mamoru Suzuki, Toshi Arima, Michihiro Sugahara, Tomoyuki | ||
| Line 5: | Line 5: | ||
<hr/> | <hr/> | ||
<b>Molecular Tour</b><br> | <b>Molecular Tour</b><br> | ||
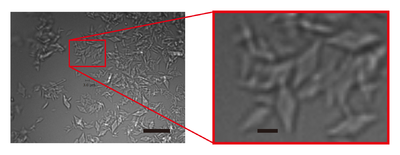
| - | Visualization of enzymatic catalysis as molecular movies may be an ultimate goal for enzymologists. However, the direct observation of structural changes associated with the ongoing reaction has been a difficult task until recently. A new method called ‘Serial Femtosecond Crystallography (SFX)’, in which protein microcrystals delivered continuously are irradiated with ultrahigh-intensity X-ray pulses produced from X-ray free-electron lasers (XFELs), is expected to be a clue for achieving such a goal. Because diffraction data can be collected before the crystals suffer from radiation damage, SFX allows the determination of X-ray damage-free structures at room temperature. The femtosecond duration of X-ray pulses is also advantageous for visualizing rapid structural changes associated with protein action. However, preparation of high-quality microcrystals with uniform size has been a bottleneck for widespread application of SFX that consumes plenty of microcrystals. This paper reports a convenient method for production of uniform, high-quality microcrystals by combining micro-seeding and batch methods. The objective protein we used is a quinoprotein metalloenzyme, copper amine oxidase from ''Arthrobacter globiformis'' (AGAO), for which high-resolution X-ray and neutron crystal structures have already been determined at cryogenic temperatures. The first X-ray-damage-free AGAO structure determined at room temperature by SFX (PDB ID: 7f8k) is herein reported. | + | [[Image:Image_1_0a.png|left|400px|thumb|'''Figure 1''' Images of AGAO microcrystals grown by combining micro-seeding and batch |
| - | The overall structure of AGAO determined by SFX had almost the same conformation as those determined previously by synchrotron X-ray and neutron crystallography performed at cryogenic temperature. The electron-density map in the active site clearly showed the resting form of the protein-derived cofactor, 2,4,5-trihydroxyphenylalanine quinone (TPQ). In addition, the active-site Cu2+ was ligated with three histidine residues and two water molecules that are located at positions identical to those determined by the previous studies. These results show that the bound Cu2+ in AGAO is resistant to X-ray photoreduction, which is accompanied by conformational changes of the metal coordination structure. The availability of high-quality microcrystals in large quantities is promising for studying the structural changes of AGAO during the catalytic reaction by the ‘mix-and-inject’ time-resolved SFX. | + | crystallisation. The right panel shows an enlarged view of the part indicated by a red rectangle in the |
| + | left panel. Scale bars in the left and right panels represent 20 and 3 µm, respectively.]] | ||
| + | {{Clear}} | ||
| + | Visualization of enzymatic catalysis as molecular movies may be an ultimate goal for enzymologists. However, the direct observation of structural changes associated with the ongoing reaction has been a difficult task until recently. A new method called ‘Serial Femtosecond Crystallography (SFX)’, in which protein microcrystals delivered continuously are irradiated with ultrahigh-intensity X-ray pulses produced from X-ray free-electron lasers (XFELs), is expected to be a clue for achieving such a goal. Because diffraction data can be collected before the crystals suffer from radiation damage, SFX allows the determination of X-ray damage-free structures at room temperature. The femtosecond duration of X-ray pulses is also advantageous for visualizing rapid structural changes associated with protein action. However, preparation of high-quality microcrystals with uniform size has been a bottleneck for widespread application of SFX that consumes plenty of microcrystals. This paper reports a convenient method for production of uniform, high-quality microcrystals by combining micro-seeding and batch methods. The objective protein we used is a quinoprotein metalloenzyme, copper amine oxidase from ''Arthrobacter globiformis'' (AGAO), for which high-resolution X-ray and neutron crystal structures have already been determined at cryogenic temperatures. The first X-ray-damage-free AGAO structure determined at room temperature by SFX (PDB ID: [[7f8k]]) is herein reported. | ||
| + | <scene name='89/891404/Cv/3'>The overall structure of AGAO determined by SFX</scene> had almost the same conformation as those determined previously by synchrotron X-ray and neutron crystallography performed at cryogenic temperature. AGAO functions as a dimer, but in the crystals obtained in this study, the unit cell contained the monomer of AGAO (shown in a green ribbon model) in the asymmetric unit. The counter subunit forming the dimer is also shown as a royal blue trace model. The electron-density map in the active site clearly showed the resting form of <scene name='89/891404/Cv/6'>the protein-derived cofactor, 2,4,5-trihydroxyphenylalanine quinone (TPQ)</scene>. In addition, the <scene name='89/891404/Cv/7'>active-site Cu2+ was ligated with three histidine residues and two water molecules</scene> that are located at positions identical to those determined by the previous studies. The assigned stick models are shown for residue 382 (TPQ) and the Cu2+ and Cu2+-ligands (His431, His433, His592, Wax, and Weq), in which Wax and Weq are the axially and equatorially Cu2+-coordinating water molecules, respectively. These results show that the bound Cu2+ in AGAO is resistant to X-ray photoreduction, which is accompanied by conformational changes of the metal coordination structure. The availability of high-quality microcrystals in large quantities is promising for studying the structural changes of AGAO during the catalytic reaction by the ‘mix-and-inject’ time-resolved SFX. | ||
| + | |||
| + | '''PDB reference:''' room-temperature structure of bacterial copper amine oxidase determined by serial femtosecond crystallography, [[7f8k]]. | ||
<b>References</b><br> | <b>References</b><br> | ||
Current revision
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.