Journal:Acta Cryst F:S2053230X21013455
From Proteopedia
(Difference between revisions)

| (11 intermediate revisions not shown.) | |||
| Line 7: | Line 7: | ||
*<scene name='89/899476/Cv/2'>Monomer of apo BpBADH</scene>, colored in rainbow from blue (N-terminus) to red (C-terminus); [[6wsa]]. | *<scene name='89/899476/Cv/2'>Monomer of apo BpBADH</scene>, colored in rainbow from blue (N-terminus) to red (C-terminus); [[6wsa]]. | ||
*<scene name='89/899476/Cv/3'>Dimer of BpBADH with NAD</scene>, monomers are shown as green and cyan ribbons, with NAD shown as ball-and-sticks; [[6wsb]]. | *<scene name='89/899476/Cv/3'>Dimer of BpBADH with NAD</scene>, monomers are shown as green and cyan ribbons, with NAD shown as ball-and-sticks; [[6wsb]]. | ||
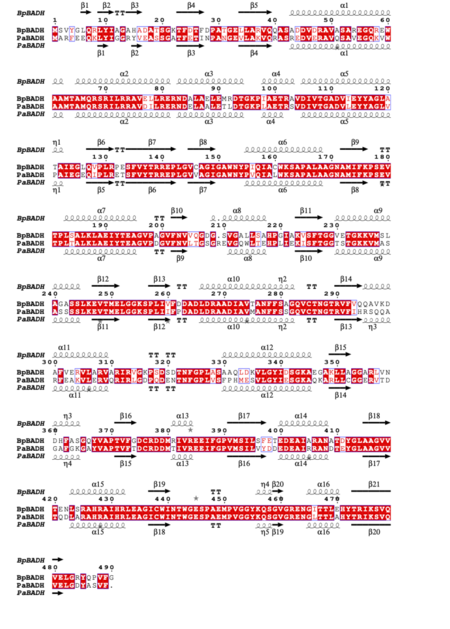
| - | The structures are similar to those of BADH from ''Pseudomonas aeruginosa'' (''Pa''BADH): | + | ''Bp''BADH has a prototypical BADH topology and shares considerable structure and sequence similarity with the ortholog from ''P. aeruginosa'' (''Pa''BADH); see static image below: |
| - | *<scene name='89/899476/Cv/ | + | [[Image:Fig2test (1).png|left|450px|thumb|Figure 2 Structural and primary sequence alignment of BpBADH and PaBADH. The secondary structure elements shown are alpha helices (α), 3<sub>10</sub>-helices (η), beta strands (β), and beta turns (TT). Identical residues are shown in white on a red background and conserved residues in red.]] |
| - | ''Pa''BADH is inhibited by the drug disulfiram which is an approved drug. Our preliminary analysis could facilitate drug repurposing studies for melioidosis. This project is an educational collaboration between the SSGCID and Hampton University. The SSGCID consortium is directed by Dr. Peter Myler (principal investigator) and comprises many different scientists working at multiple centers towards determining the three-dimensional structures of proteins from biodefense organisms and emerging infectious diseases. Dylan K. Beard was part of a pilot Hampton University Chemistry Education and Mentorship Course-based undergraduate research (HU-ChEM CURES) funded by the NIGMS. | + | {{Clear}} |
| + | |||
| + | The structures are similar to those of BADH from ''Pseudomonas aeruginosa'' (''Pa''BADH). The co-factor binding domains of ''Bp''BADH ([[6wsb]]) and ''Pa''BADH ([[4caz]]) are well conserved (identical residues of both structures are labeled in green, while non-identical in red): | ||
| + | *<scene name='89/899476/Cv/11'>Co-factor binding domain of BpBADH</scene> ([[6wsb]]). | ||
| + | *<scene name='89/899476/Cv/10'>Co-factor binding domain of PaBADH</scene> ([[4caz]]). | ||
| + | *<scene name='89/899476/Cv/12'>Superposition of BpBADH and PaBADH</scene> (BpBADH is colored in green, PaBADH is in olive). | ||
| + | ''Pa''BADH is inhibited by the drug disulfiram which is an approved drug. Both structures show the <scene name='89/899476/Cv/13'>conserved catalytic cysteine</scene> irreversibly inhibited by disulfiram. Our preliminary analysis could facilitate drug repurposing studies for melioidosis. This project is an educational collaboration between the SSGCID and Hampton University. The SSGCID consortium is directed by Dr. Peter Myler (principal investigator) and comprises many different scientists working at multiple centers towards determining the three-dimensional structures of proteins from biodefense organisms and emerging infectious diseases. Dylan K. Beard was part of a pilot Hampton University Chemistry Education and Mentorship Course-based undergraduate research (HU-ChEM CURES) funded by the NIGMS. | ||
| + | |||
| + | '''PDB references:''' betaine aldehyde dehydrogenase, [[6wsa]]; bound to cofactor, [[6wsb]]. | ||
<b>References</b><br> | <b>References</b><br> | ||
Current revision
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.