Journal:Acta Cryst D:S2059798322008373
From Proteopedia
(Difference between revisions)

| Line 4: | Line 4: | ||
<hr/> | <hr/> | ||
<b>Molecular Tour</b><br> | <b>Molecular Tour</b><br> | ||
| - | Crystal structure of bacterial nitroreductase (NR) NfsB in complex with the traditional medicine berberine (BBR) showed <scene name='92/920253/Cv/2'>BBR binds into the active pocket at the NfsB dimer interface</scene>. BBR is mainly stabilized by π-stacking interactions with both neighboring aromatic residues and the cofactor FMN. Several well-ordered water molecules neighboring BBR in the active site probably donate protons in conjunction with electron transfer from FMN for BBR reduction. | + | Crystal structure of bacterial nitroreductase (NR) NfsB in complex with the traditional medicine berberine (BBR) showed <scene name='92/920253/Cv/2'>BBR binds into the active pocket at the NfsB dimer interface</scene>. BBR is mainly stabilized by <scene name='92/920253/Cv/3'>π-stacking interactions with both neighboring aromatic residues and the cofactor FMN</scene>. Several well-ordered water molecules neighboring BBR in the active site probably donate protons in conjunction with electron transfer from FMN for BBR reduction. |
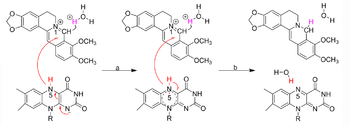
[[Image:NRpathway.png|left|350px|thumb|A proposed mechanism of BBR-to-dhBBR conversion by bacterial NRs]] | [[Image:NRpathway.png|left|350px|thumb|A proposed mechanism of BBR-to-dhBBR conversion by bacterial NRs]] | ||
{{Clear}} | {{Clear}} | ||
Revision as of 07:08, 24 August 2022
| |||||||||||
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.