Beta-adrenergic receptor kinase
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 1: | Line 1: | ||
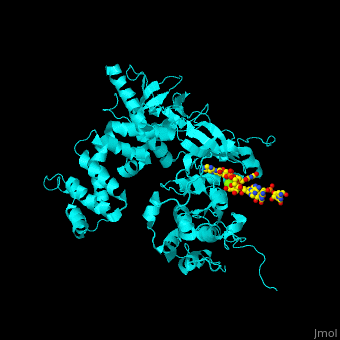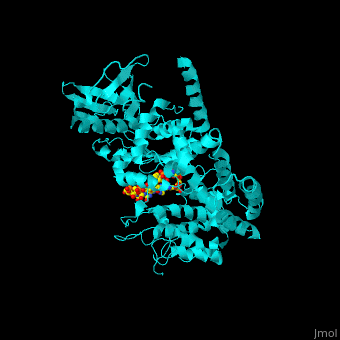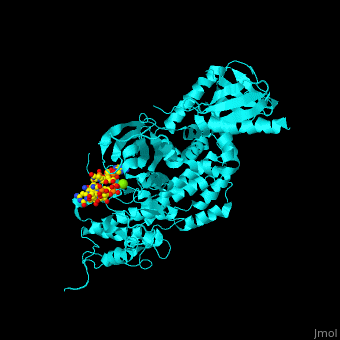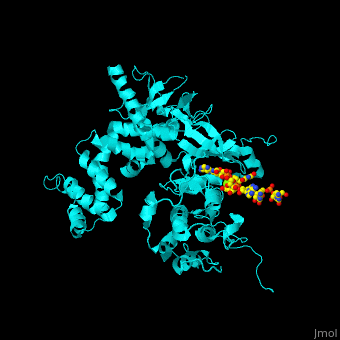
<StructureSection load='' size='350' side='right' caption='Human BARK1 complex with RNA and Mg+2 ion (green) (PDB code [[3uzt]])' scene='70/705677/Cv/1'> | <StructureSection load='' size='350' side='right' caption='Human BARK1 complex with RNA and Mg+2 ion (green) (PDB code [[3uzt]])' scene='70/705677/Cv/1'> | ||
| - | '''Beta adrenergic receptor kinase''' (BARK), currently termed GRK2 for G protein-coupled receptor kinase 2, is an intracellular serine/threonine kinase. BARK is activated by protein kinase A. BARK phosphorylates the β | + | '''Beta adrenergic receptor kinase''' or '''G-protein coupled receptor kinase''' (BARK), currently termed GRK2 for G protein-coupled receptor kinase 2, is an intracellular serine/threonine kinase. BARK is activated by protein kinase A. BARK phosphorylates the β adrenergic receptor (BAR) as well as many other G protein-coupled receptors when they are occupied by the agonist thus causing their desensitization. BARK belongs to the family of G protein-coupled receptor kinases (GRK) that encompases 5 different isozymes. <ref>PMID:2871555</ref> |
<scene name='70/705677/Cv/4'>Human BARK1 complex with RNA and Mg+2 ion</scene>; | <scene name='70/705677/Cv/4'>Human BARK1 complex with RNA and Mg+2 ion</scene>; | ||
Current revision
| |||||||||||
References
- ↑ Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797-801. PMID:2871555
- ↑ Tesmer VM, Lennarz S, Mayer G, Tesmer JJ. Molecular Mechanism for Inhibition of G Protein-Coupled Receptor Kinase 2 by a Selective RNA Aptamer. Structure. 2012 Jun 21. PMID:22727813 doi:10.1016/j.str.2012.05.002
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Cristina Murga, Joel L. Sussman