Acetylcholinesterase
From Proteopedia
(Difference between revisions)
| (21 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
[[Image:small_wh_ray0001.gif|left|150px]]<br /> | [[Image:small_wh_ray0001.gif|left|150px]]<br /> | ||
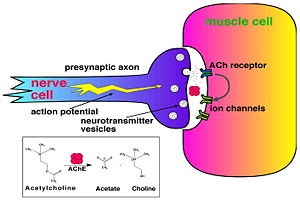
'''Acetylcholinesterase''' (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, [[Acetylcholine|acetylcholine]] (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors. | '''Acetylcholinesterase''' (AChE) is key enzyme in the nervous system of animals. By rapid hydrolysis of the neurotransmitter, [[Acetylcholine|acetylcholine]] (ACh), AChE terminates neurotransmission at cholinergic synapses. It is a very fast enzyme, especially for a serine hydrolase, functioning at a rate approaching that of a diffusion-controlled reaction. AChE inhibitors are among the key drugs approved by the FDA for management of Alzheimer's disease (AD). The powerful toxicity of organophosphorus (OP) poisons is attributed primarily to their potent AChE inhibitors. | ||
| + | |||
| + | See also [[Acetylcholinesterase (Hebrew)]] | ||
== Key Enzyme in the Nervous System == | == Key Enzyme in the Nervous System == | ||
| Line 9: | Line 11: | ||
[[Image:Synapse_Schematic.jpg|thumb|Cholinergic Synapse|300px|left]] | [[Image:Synapse_Schematic.jpg|thumb|Cholinergic Synapse|300px|left]] | ||
| - | [http://en.wikipedia.org/wiki/Acetylcholinesterase Acetylcholinesterase] [http://en.wikipedia.org/wiki/Hydrolysis hydrolysizes] the [http://en.wikipedia.org/wiki/Neurotransmitter neurotransmitter] [http://en.wikipedia.org/wiki/Acetylcholine acetylcholine] <scene name='2ace/Cv/2'>(ACh)</scene>, producing <scene name='2ace/Cv/3'>choline and an acetate</scene> group. ACh directly binds <scene name='22/22/Cv/1'>Ser200</scene> (via its [http://en.wikipedia.org/wiki/Nucleophile nucleophilic] Oγ atom) within the <scene name='2ace/Cv/5'>catalytic triad (Ser200, His440, and Glu327)</scene> (ACh/''Tc''AChE structure [[2ace]]). The residues <scene name='2ace/Cv/6'>Trp84 and Phe330</scene> are also important in the [http://en.wikipedia.org/wiki/Ligand ligand] recognition <ref name="Raves">PMID:8989325</ref>. After this binding acetylcholinesterase <scene name='2ace/Cv/7'>hydrolysizes</scene> ACh. | + | [http://en.wikipedia.org/wiki/Acetylcholinesterase Acetylcholinesterase] [http://en.wikipedia.org/wiki/Hydrolysis hydrolysizes] the [http://en.wikipedia.org/wiki/Neurotransmitter neurotransmitter] [http://en.wikipedia.org/wiki/Acetylcholine acetylcholine] <scene name='2ace/Cv/2'>(ACh)</scene>, producing <scene name='2ace/Cv/3'>choline and an acetate</scene> group. ACh directly binds <scene name='22/22/Cv/1'>Ser200</scene> (via its [http://en.wikipedia.org/wiki/Nucleophile nucleophilic] Oγ atom) within the <scene name='2ace/Cv/5'>catalytic triad (Ser200, His440, and Glu327)</scene> (ACh/''Tc''AChE structure [[2ace]]). The residues <scene name='2ace/Cv/6'>Trp84 and Phe330</scene> are also important in the [http://en.wikipedia.org/wiki/Ligand ligand] recognition <ref name="Raves">PMID:8989325</ref>. After this binding acetylcholinesterase <scene name='2ace/Cv/7'>hydrolysizes</scene> ACh. <br /> |
| - | + | See also [[Acetylcholinesterase with acetylcholine]]. | |
| - | + | ||
== Treatment of Alzheimer's disease == | == Treatment of Alzheimer's disease == | ||
| - | [http://en.wikipedia.org/wiki/Alzheimer's_disease Alzheimer's disease] (AD) is a disorder that attacks the [http://en.wikipedia.org/wiki/Central_nervous_system central nervous system] through progressive degeneration of its neurons. AD occurs in around 10% of the elderly and, as yet, there is no known cure. Patients with this disease develop [http://en.wikipedia.org/wiki/Dementia dementia] which becomes more severe as the disease progresses. It was suggested that symptoms of AD are caused by decrease of activity of [http://en.wikipedia.org/wiki/Cholinergic cholinergic] [http://en.wikipedia.org/wiki/Neocortex neocortical] and [http://en.wikipedia.org/wiki/Hippocampus hippocampal] neurons. Treatment of AD by ACh precursors and [http://en.wikipedia.org/wiki/Cholinergic cholinergic] [http://en.wikipedia.org/wiki/Agonist agonists] was ineffective or caused severe side effects. ACh hydrolysis by AChE causes termination of cholinergic neurotransmission. Therefore, compounds which inhibit AChE might significantly increase the levels of ACh depleted in AD. Indeed, it was shown that [http://en.wikipedia.org/wiki/Acetylcholinesterase_inhibitor AChE inhibitors] improve the cognitive abilities of AD patients at early stages of the disease development. <br /> | + | [http://en.wikipedia.org/wiki/Alzheimer's_disease Alzheimer's disease] (AD) is a disorder that attacks the [http://en.wikipedia.org/wiki/Central_nervous_system central nervous system] through progressive degeneration of its neurons. AD occurs in around 10% of the elderly and, as yet, there is no known cure. Patients with this disease develop [http://en.wikipedia.org/wiki/Dementia dementia] which becomes more severe as the disease progresses. It was suggested that symptoms of AD are caused by decrease of activity of [http://en.wikipedia.org/wiki/Cholinergic cholinergic] [http://en.wikipedia.org/wiki/Neocortex neocortical] and [http://en.wikipedia.org/wiki/Hippocampus hippocampal] neurons. Treatment of AD by ACh precursors and [http://en.wikipedia.org/wiki/Cholinergic cholinergic] [http://en.wikipedia.org/wiki/Agonist agonists] was ineffective or caused severe side effects. ACh hydrolysis by AChE causes termination of cholinergic neurotransmission. Therefore, compounds which inhibit AChE might significantly increase the levels of ACh depleted in AD. Indeed, it was shown that [http://en.wikipedia.org/wiki/Acetylcholinesterase_inhibitor AChE inhibitors] improve the cognitive abilities of AD patients at early stages of the disease development. The way in which the various cholinesterase inhibitors interact with AChE can be see at:<br /> |
| - | + | *[[Acetylcholinesterase: Treatment of Alzheimer's disease]].<br /> | |
| + | *[[Acetylcholinesterase complexed with N-9-(1',2',3',4'-tetrahydroacridinyl)-1,8-diaminooctane]].<br /> | ||
== Organophosphorus acid anhydride nerve agents == | == Organophosphorus acid anhydride nerve agents == | ||
| Line 23: | Line 25: | ||
As was mentioned above, AChE hydrolysizes the neurotransmitter <scene name='2wfz/Al/2'>ACh</scene>, producing <scene name='2wfz/Al/3'>choline and an acetate</scene> group. <scene name='2wfz/Al/2'>ACh</scene> directly binds catalytic <scene name='2wfz/Al/4'>Ser200</scene> (via its nucleophilic Oγ atom). <scene name='2wfz/Al/5'>Soman</scene>, [http://en.wikipedia.org/wiki/Soman O-(1,2,2-trimethylpropyl) methylphosphonofluoridate] (<font color='violet'><b>fluorine atom is colored violet</b></font> and <font color='darkmagenta'><b>phosphorus atom is colored darkmagenta</b></font>), is one of the most toxic OPs. Soman inhibits AChE by <scene name='2wfz/Al/6'>covalent binding</scene> to catalytic Ser200, <scene name='2wfz/Al/7'>mimicking ACh</scene>. This process <scene name='2wfz/Al/8'>(phosphonylation)</scene> implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the <scene name='2wfz/Al/9'>dealkylation</scene> ("aging") of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of <scene name='2wfz/Al/8'>phosphonylation</scene>, AChE can be <scene name='2wfz/Al/11'>reactivated</scene> by nucleophiles, such as pralidoxime (2-PAM), resulting in <scene name='2wfz/Al/12'>cleavage</scene> of the phosphonyl adduct from Ser200 Oγ. | As was mentioned above, AChE hydrolysizes the neurotransmitter <scene name='2wfz/Al/2'>ACh</scene>, producing <scene name='2wfz/Al/3'>choline and an acetate</scene> group. <scene name='2wfz/Al/2'>ACh</scene> directly binds catalytic <scene name='2wfz/Al/4'>Ser200</scene> (via its nucleophilic Oγ atom). <scene name='2wfz/Al/5'>Soman</scene>, [http://en.wikipedia.org/wiki/Soman O-(1,2,2-trimethylpropyl) methylphosphonofluoridate] (<font color='violet'><b>fluorine atom is colored violet</b></font> and <font color='darkmagenta'><b>phosphorus atom is colored darkmagenta</b></font>), is one of the most toxic OPs. Soman inhibits AChE by <scene name='2wfz/Al/6'>covalent binding</scene> to catalytic Ser200, <scene name='2wfz/Al/7'>mimicking ACh</scene>. This process <scene name='2wfz/Al/8'>(phosphonylation)</scene> implicates nucleophilic attack of the Ser200 nucleophilic Oγ atom on the phosphorus atom of soman, with concomitant departure of its fluoride atom. After that AChE catalyzes the <scene name='2wfz/Al/9'>dealkylation</scene> ("aging") of the soman or other OP. This causes irreversible inhibition of AChE, "aged" soman/AChE conjugate can not be reactivated. However, before “aging”, at the step of <scene name='2wfz/Al/8'>phosphonylation</scene>, AChE can be <scene name='2wfz/Al/11'>reactivated</scene> by nucleophiles, such as pralidoxime (2-PAM), resulting in <scene name='2wfz/Al/12'>cleavage</scene> of the phosphonyl adduct from Ser200 Oγ. | ||
At the <scene name='2wfz/Ali/3'>active site of the nonaged soman/TcAChE conjugate</scene> ([[2wfz]]) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a <font color='red'><b>red ball</b></font>. The active site residues of the nonaged soman/TcAChE are colored <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span>. The O2 atom of the <scene name='2wfz/Ali/4'>dealkylated (aged) soman</scene> ([[2wg0]]) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored <span style="color:pink;background-color:black;font-weight:bold;">pink</span>. <scene name='2wfz/Ali/5'>Alignment</scene> of the structures of the nonaged ([[2wfz]]) and aged ([[2wg0]]) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an <scene name='2wfz/Ali/7'>unfavorable and nonfunctional conformation</scene> after soman aging to ''Tc''AChE ([[2wg1]]) <ref name="Sanson">PMID:19642642</ref>. | At the <scene name='2wfz/Ali/3'>active site of the nonaged soman/TcAChE conjugate</scene> ([[2wfz]]) the catalytic His440 forms hydrogen bonds with Ser200 Oγ and Glu327 Oε1 via its Nε2 and Nδ1 nitrogens, respectively. The O2 atom of soman is within hydrogen bonding distance of His440 Nε2. Soman O1 mimicks carbonyl oxygen of ACh. A water molecule 1001 interacting with soman O2 is represented as a <font color='red'><b>red ball</b></font>. The active site residues of the nonaged soman/TcAChE are colored <span style="color:yellow;background-color:black;font-weight:bold;">yellow</span>. The O2 atom of the <scene name='2wfz/Ali/4'>dealkylated (aged) soman</scene> ([[2wg0]]) forms a salt bridge with His440 Nε2. The active site residues of the aged soman/TcAChE are colored <span style="color:pink;background-color:black;font-weight:bold;">pink</span>. <scene name='2wfz/Ali/5'>Alignment</scene> of the structures of the nonaged ([[2wfz]]) and aged ([[2wg0]]) conjugates reveals a small, but important, change within the active site - the imidazole ring of His440 is tilted back to a native-like conformation after dealkylation. The water molecule 1001, which interacts with soman O2 in the nonaged crystal structure, is not within hydrogen bonding distance of O2 in the aged crystal structure. 2-PAM binds poorly to the nonaged phosphonylated enzyme (its electron density was not found) and binds in an <scene name='2wfz/Ali/7'>unfavorable and nonfunctional conformation</scene> after soman aging to ''Tc''AChE ([[2wg1]]) <ref name="Sanson">PMID:19642642</ref>. | ||
| - | |||
| - | |||
| - | |||
| - | |||
==Additional resources== | ==Additional resources== | ||
| - | + | see: [[Acetylcholinesterase_Additional_Resources]] | |
| - | + | ||
| - | + | ||
| - | [ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ==Movies== | |
| - | + | see: [[Acetylcholinesterase_Movies]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Acetylcholinesterase 3D structures== | ==Acetylcholinesterase 3D structures== | ||
| - | See [[Acetylcholinesterase 3D structures]] | ||
| - | |||
| + | [[Acetylcholinesterase 3D structures]] | ||
==References== | ==References== | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, David Canner, Eran Hodis, Clifford Felder, Jaime Prilusky, Harry Greenblatt, Yechun Xu