We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ricin: Structure and function
From Proteopedia
(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
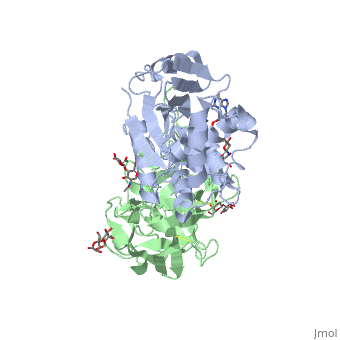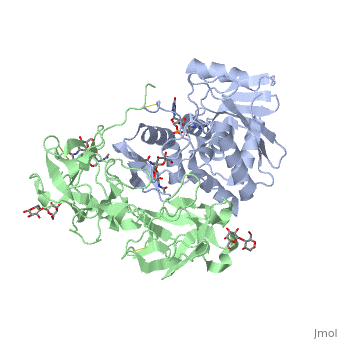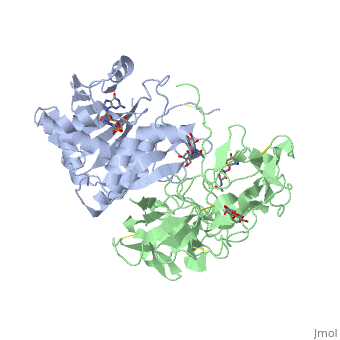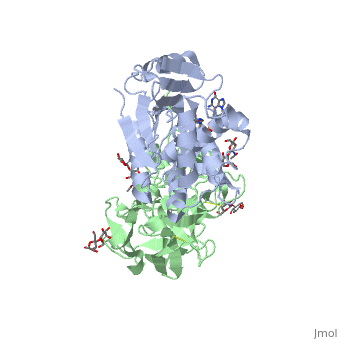
| - | <StructureSection load='3rtj' size='350' side='right' caption='Structure of | + | <StructureSection load='3rtj' size='350' side='right' caption='Structure of glycosylated ricin toxin (green and grey) complex with RNA dinucleotide (yellow) (PDB code [[3rtj]])' scene=''> |
__TOC__ | __TOC__ | ||
| - | ==Ricin== | + | ==Ricin Overview== |
Found inside of the seeds of the castor oil plant and in castor beans, the cytotoxin, <scene name='91/910715/Ricin/1'>Ricin</scene>, is known to be one of Earth's most toxic proteins. Ricin was officially discovered in the year 1888 during the investigation of castor seed toxicity, which had been a mystery to scientists since their observations of its effects in the late nineteenth century. German scientist, Peter Hermann Stillmark, was able to extract this compound from the castor seed and purify it into the structure we see today. Originally thought to be used in aiding cancer patients, its highly toxic nature to humans deemed it too dangerous to use in a medical setting. Doctors and scientists have even worked together to pick apart the protein and use each chain in cancer therapy separately. On the other hand, it’s toxicity attracted the attention of criminals, military, and terrorists who came up with the idea of using it as a weapon in bio-terroristic attacks<ref>Etimad, L., Moshiri, M., & Hamid, F. (2019, June 6). Ricin: An ancient story for a timeless plant toxin. RBMB. Retrieved April 23, 2022,</ref>. | Found inside of the seeds of the castor oil plant and in castor beans, the cytotoxin, <scene name='91/910715/Ricin/1'>Ricin</scene>, is known to be one of Earth's most toxic proteins. Ricin was officially discovered in the year 1888 during the investigation of castor seed toxicity, which had been a mystery to scientists since their observations of its effects in the late nineteenth century. German scientist, Peter Hermann Stillmark, was able to extract this compound from the castor seed and purify it into the structure we see today. Originally thought to be used in aiding cancer patients, its highly toxic nature to humans deemed it too dangerous to use in a medical setting. Doctors and scientists have even worked together to pick apart the protein and use each chain in cancer therapy separately. On the other hand, it’s toxicity attracted the attention of criminals, military, and terrorists who came up with the idea of using it as a weapon in bio-terroristic attacks<ref>Etimad, L., Moshiri, M., & Hamid, F. (2019, June 6). Ricin: An ancient story for a timeless plant toxin. RBMB. Retrieved April 23, 2022,</ref>. | ||
| Line 11: | Line 11: | ||
== Structure == | == Structure == | ||
The structure of Ricin consists of two polypeptide chains known as the A and B chain that carry out different functions within the protein due to their amino acid sequences. This heterodimer’s chains are joined by disulfide bonds and have fairly similar molecular weights. In order for ricin to carry out its function, the two chains need to be linked, although they do separate further into the mechanism of action. | The structure of Ricin consists of two polypeptide chains known as the A and B chain that carry out different functions within the protein due to their amino acid sequences. This heterodimer’s chains are joined by disulfide bonds and have fairly similar molecular weights. In order for ricin to carry out its function, the two chains need to be linked, although they do separate further into the mechanism of action. | ||
| - | <scene name='91/910715/Achain/1'>The A chain</scene> (Ricin A) is a globular protein that consists of 8 beta sheets and 8 alpha helices. It is composed of 267 amino acids and is separated into three structural domains. The first domain is made up of parallel and antiparallel beta sheets, the second contains alpha helices, and the third consists of both | + | <scene name='91/910715/Achain/1'>The A chain</scene> (Ricin A) is a globular protein that consists of 8 beta sheets and 8 alpha helices. It is composed of 267 amino acids and is separated into three structural domains. The first domain is made up of parallel and antiparallel beta sheets, the second contains alpha helices, and the third consists of both. These three domains carry out the function of ribosome inactivation within the Golgi apparatus in a cell, as the active site for depurination lies on the second alpha helical domain. The enzymatic activity of this protein is mainly seen on this chain in the ricin loop, where an adenine of a target cell becomes trapped by two tyrosines. This will result in the prevention of mRNA translation. |
| - | The B chain (Ricin B) is a lectin that is composed of 262 amino acids and carries out the action of binding through a galactose binding terminal. This chain does not have any alpha helical or beta sheet structure but does fold into two domains. | + | <scene name='91/910715/Bchain/1'>The B chain</scene> (Ricin B) is a lectin that is composed of 262 amino acids and carries out the action of binding through a galactose binding terminal. This chain does not have any alpha helical or beta sheet structure but does fold into two domains. |
| - | These chains are not exclusive to castor plants and they can be found in other non toxic vegetation as well; it is only when these two chains are bound together where they are able to have devastating impacts on an organism<ref>Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin Poisoning: A Comprehensive Review. JAMA. 2005;294(18):2342–2351. doi:10.1001/jama.294.18.2342</ref>. | + | These chains are not exclusive to castor plants and they can be found in other non toxic vegetation as well; it is only when these two chains are bound together where they are able to have devastating impacts on an organism<ref>Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J. 1994 Feb;8(2):201-8. PMID: 8119491.</ref><ref>Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin Poisoning: A Comprehensive Review. JAMA. 2005;294(18):2342–2351. doi:10.1001/jama.294.18.2342</ref>. |
== Mechanism of Action == | == Mechanism of Action == | ||
When the A chain and B chain are joined, they are able to work together to carry out enzymatic activity within an organism. Because they have two completely different properties, their individual functions work together. The main goal of ricin is to inhibit protein synthesis, a process that heavily relies on organelles called ribosomes. When these ribosomes are damaged or inactivated, this halts protein synthesis within a cell. | When the A chain and B chain are joined, they are able to work together to carry out enzymatic activity within an organism. Because they have two completely different properties, their individual functions work together. The main goal of ricin is to inhibit protein synthesis, a process that heavily relies on organelles called ribosomes. When these ribosomes are damaged or inactivated, this halts protein synthesis within a cell. | ||
| - | At the start of the process, the B chain’s purpose is to gain entry into a target cell so that the A chain can carry out ribosomal deactivation. As Ricin B attaches to the galactose residues on a cell membrane, the protein is able to invade the cell through the cleavage of the cell membrane, endocytosis. Now that the B chain has done its part in the protein’s function, Chain A has entry into the cytosol of the cell | + | At the start of the process, the B chain’s purpose is to gain entry into a target cell so that the A chain can carry out ribosomal deactivation. As Ricin B attaches to the galactose residues on a cell membrane, the protein is able to invade the cell through the cleavage of the cell membrane, endocytosis. Now that the B chain has done its part in the protein’s function, Chain A has entry into the cytosol of the cell. |
| - | After Ricin enters the cell, the disulfide bond between the A and B chains is cleaved and the A chain is released into the cell, working its way to the Golgi network. Ricin A functions to deactivate ribosomal activity through elongation. The enzymatic activity of this protein can inactivate thousands of ribosomes per minute, making it a very fast acting protein. As stated in the structure of the protein, there is a ricin loop on chain A where two tyrosine residues play an important role in the deactivation of the ribosome. <scene name='91/910715/Tyr80_123/1'>Tyr80 and Tyr123</scene> sandwich an adenine to inhibit further protein synthesis. After protein production in a cell is stopped, it is unclear whether the two chains link back together and move on to another cell or whether other cells are affected by new ricin proteins<ref>PMID: 8119491.</ref>. | + | After Ricin enters the cell, the disulfide bond between the A and B chains is cleaved and the A chain is released into the cell, working its way to the Golgi network. Ricin A functions to deactivate ribosomal activity through elongation. The enzymatic activity of this protein can inactivate thousands of ribosomes per minute, making it a very fast acting protein. As stated in the structure of the protein, there is a ricin loop on chain A where two tyrosine residues play an important role in the deactivation of the ribosome. <scene name='91/910715/Tyr80_123/1'>Tyr80 and Tyr123</scene> sandwich an adenine to inhibit further protein synthesis. After protein production in a cell is stopped, it is unclear whether the two chains link back together and move on to another cell or whether other cells are affected by new ricin proteins<ref>Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J. 1994 Feb;8(2):201-8. PMID: 8119491.</ref>. |
== Ricin Poisoning: Symptoms == | == Ricin Poisoning: Symptoms == | ||
Now that we understand the structure and mechanism of action behind Ricin, how can we make sure we take the correct steps in identifying if you may have been exposed to this protein? The main mechanism of this protein is its ability to halt protein synthesis. Because it doesn’t damage the cell directly and instead stops it’s functioning, the symptoms of ricin poisoning can take up to a couple days to be noticed, depending on exposure and dose. | Now that we understand the structure and mechanism of action behind Ricin, how can we make sure we take the correct steps in identifying if you may have been exposed to this protein? The main mechanism of this protein is its ability to halt protein synthesis. Because it doesn’t damage the cell directly and instead stops it’s functioning, the symptoms of ricin poisoning can take up to a couple days to be noticed, depending on exposure and dose. | ||
Current revision
| |||||||||||
References
- ↑ Etimad, L., Moshiri, M., & Hamid, F. (2019, June 6). Ricin: An ancient story for a timeless plant toxin. RBMB. Retrieved April 23, 2022,
- ↑ Tumer, N. E. (2019). Introduction to the Toxins Special Issue “Ricin Toxins.” Toxins, 12(1).
- ↑ Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J. 1994 Feb;8(2):201-8. PMID: 8119491.
- ↑ Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin Poisoning: A Comprehensive Review. JAMA. 2005;294(18):2342–2351. doi:10.1001/jama.294.18.2342
- ↑ Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. FASEB J. 1994 Feb;8(2):201-8. PMID: 8119491.
- ↑ Gal, Y., Mazor, O., Falach, R., Sapoznikov, A., Kronman, C., & Sabo, T. (2017). Treatments for Pulmonary Ricin Intoxication: Current Aspects and Future Prospects. Toxins, 9(10).