Dimethylarginine Dimethylaminohydrolase
From Proteopedia
(Difference between revisions)
| Line 47: | Line 47: | ||
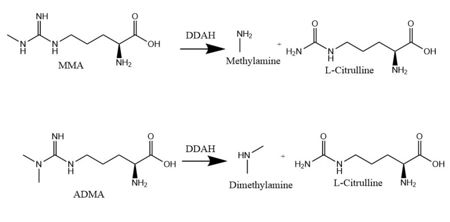
DDAH works to hydrolyze MMA and ADMA <ref name="frey" />. Both MMA and ADMA competitively inhibit NO synthesis by inhibiting Nitric Oxide Synthase (NOS). NO is made by NOS creating L-citrulline from <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine L-arginine]</span> <ref name="frey" />. If DDAH is overexpressed, NOS activity will subsequently increase <ref name="frey" />. ADMA and MMA can <span class="plainlinks">[https://en.wikipedia.org/wiki/Enzyme_inhibitor inhibit]</span> the synthesis of NO by competitively inhibiting all three kinds of NOS (endothelial, neuronal, and inducible) <ref name="frey" />. Underexpression or inhibition of DDAH decreases NOS activity and NO levels will decrease. Because of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide nitric oxide’s (NO)]</span> role in signaling and defense, NO levels in an organism must be regulated to reduce damage to cells <ref name="janssen">Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/23097221 23097221]</span> doi:<span class="plainlinks">[http://onlinelibrary.wiley.com/doi/10.1002/path.4127/references;jsessionid=C34C6C633A21C2ECE14278BBC902AD71.f03t04?globalMessage=0 10.1002/path.4127]</span></ref>. NO is an important signaling and effector molecule in <span class="plainlinks">[https://en.wikipedia.org/wiki/Neurotransmission neurotransmission]</span>, bacterial defense, and regulation of vascular tone <ref name="colasanti">Colasanti M, Suzuki H. The dual personality of NO. ScienceDirect. 2000 Jul 1;21(7):249-252. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/10979862 10979862]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0165614700014991 10.1016/S0165-6147(00)01499-1]</span></ref>. Because NO is highly toxic, freely diffusible across membranes, and its radical form is fairly reactive, cells must maintain a large control on concentrations by regulating NOS activity and the activity of enzymes such as DDAH that have an indirect effect of the concentration of NO <ref name="rassaf">Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Rad. Biol. Med. 2004 Feb 15;36(4):413-422. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14975444 14975444]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0891584903007962 10.1016/j.freeradbiomed.2003.11.011]</span></ref>. An imbalance of NO contributes to several diseases. Low NO levels, potentially caused by low DDAH activity and therefore high MMA and ADMA concentrations, have been associated with diseases such as <span class="plainlinks">[https://en.wikipedia.org/wiki/Uremia uremia]</span>, <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/heart-failure/basics/definition/con-20029801 chronic heart failure]</span>, <span class="plainlinks">[https://en.wikipedia.org/wiki/Atherosclerosis atherosclerosis]</span>, and <span class="plainlinks">[https://en.wikipedia.org/wiki/Hyperhomocysteinemia hyperhomocysteinemia]</span> <ref name="tsao">Tsao PS, Cooke JP. Endothelial alterations in hypercholesterolemia: more than simply vasodilator dysfunction. Journal of Cardiovascular Pharmacology. 1998;32(3):48-53. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/9883748 9883748]</span></ref>. High levels of NO have been involved with diseases such as <span class="plainlinks">[https://en.wikipedia.org/wiki/Septic_shock septic shock]</span>, <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/migraine-headache/home/ovc-20202432 migraine]</span>, <span class="plainlinks">[https://en.wikipedia.org/wiki/Inflammation inflammation]</span>, and <span class="plainlinks">[https://en.wikipedia.org/wiki/Neurodegeneration neurodegenerative disorders]</span> <ref name="vallance">Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat. Rev. Drug Discov. 2002 Dec;1(12):939-950. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/12461516 12461516]</span> doi:<span class="plainlinks">[http://www.nature.com/nrd/journal/v1/n12/full/nrd960.html 10.1038/nrd960]</span></ref>. Because of the effects on NO levels and known inhibitors to DDAH, regulation of DDAH may be an effective way to regulate NO levels, therefore treating these diseases <ref name="frey" />. Additionally, researchers can take advantage of the fact that there are two different isoforms of this enzyme and create drugs that target one isoform over another to control NO levels in specific tissues in the body <ref name="frey" />. | DDAH works to hydrolyze MMA and ADMA <ref name="frey" />. Both MMA and ADMA competitively inhibit NO synthesis by inhibiting Nitric Oxide Synthase (NOS). NO is made by NOS creating L-citrulline from <span class="plainlinks">[https://en.wikipedia.org/wiki/Arginine L-arginine]</span> <ref name="frey" />. If DDAH is overexpressed, NOS activity will subsequently increase <ref name="frey" />. ADMA and MMA can <span class="plainlinks">[https://en.wikipedia.org/wiki/Enzyme_inhibitor inhibit]</span> the synthesis of NO by competitively inhibiting all three kinds of NOS (endothelial, neuronal, and inducible) <ref name="frey" />. Underexpression or inhibition of DDAH decreases NOS activity and NO levels will decrease. Because of <span class="plainlinks">[https://en.wikipedia.org/wiki/Nitric_oxide nitric oxide’s (NO)]</span> role in signaling and defense, NO levels in an organism must be regulated to reduce damage to cells <ref name="janssen">Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/23097221 23097221]</span> doi:<span class="plainlinks">[http://onlinelibrary.wiley.com/doi/10.1002/path.4127/references;jsessionid=C34C6C633A21C2ECE14278BBC902AD71.f03t04?globalMessage=0 10.1002/path.4127]</span></ref>. NO is an important signaling and effector molecule in <span class="plainlinks">[https://en.wikipedia.org/wiki/Neurotransmission neurotransmission]</span>, bacterial defense, and regulation of vascular tone <ref name="colasanti">Colasanti M, Suzuki H. The dual personality of NO. ScienceDirect. 2000 Jul 1;21(7):249-252. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/10979862 10979862]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0165614700014991 10.1016/S0165-6147(00)01499-1]</span></ref>. Because NO is highly toxic, freely diffusible across membranes, and its radical form is fairly reactive, cells must maintain a large control on concentrations by regulating NOS activity and the activity of enzymes such as DDAH that have an indirect effect of the concentration of NO <ref name="rassaf">Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Rad. Biol. Med. 2004 Feb 15;36(4):413-422. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/14975444 14975444]</span> doi:<span class="plainlinks">[http://www.sciencedirect.com/science/article/pii/S0891584903007962 10.1016/j.freeradbiomed.2003.11.011]</span></ref>. An imbalance of NO contributes to several diseases. Low NO levels, potentially caused by low DDAH activity and therefore high MMA and ADMA concentrations, have been associated with diseases such as <span class="plainlinks">[https://en.wikipedia.org/wiki/Uremia uremia]</span>, <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/heart-failure/basics/definition/con-20029801 chronic heart failure]</span>, <span class="plainlinks">[https://en.wikipedia.org/wiki/Atherosclerosis atherosclerosis]</span>, and <span class="plainlinks">[https://en.wikipedia.org/wiki/Hyperhomocysteinemia hyperhomocysteinemia]</span> <ref name="tsao">Tsao PS, Cooke JP. Endothelial alterations in hypercholesterolemia: more than simply vasodilator dysfunction. Journal of Cardiovascular Pharmacology. 1998;32(3):48-53. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/9883748 9883748]</span></ref>. High levels of NO have been involved with diseases such as <span class="plainlinks">[https://en.wikipedia.org/wiki/Septic_shock septic shock]</span>, <span class="plainlinks">[http://www.mayoclinic.org/diseases-conditions/migraine-headache/home/ovc-20202432 migraine]</span>, <span class="plainlinks">[https://en.wikipedia.org/wiki/Inflammation inflammation]</span>, and <span class="plainlinks">[https://en.wikipedia.org/wiki/Neurodegeneration neurodegenerative disorders]</span> <ref name="vallance">Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat. Rev. Drug Discov. 2002 Dec;1(12):939-950. PMID:<span class="plainlinks">[https://www.ncbi.nlm.nih.gov/pubmed/12461516 12461516]</span> doi:<span class="plainlinks">[http://www.nature.com/nrd/journal/v1/n12/full/nrd960.html 10.1038/nrd960]</span></ref>. Because of the effects on NO levels and known inhibitors to DDAH, regulation of DDAH may be an effective way to regulate NO levels, therefore treating these diseases <ref name="frey" />. Additionally, researchers can take advantage of the fact that there are two different isoforms of this enzyme and create drugs that target one isoform over another to control NO levels in specific tissues in the body <ref name="frey" />. | ||
| - | + | ||
== 3D Structures of dimethylarginine dimethylaminohydrolase 1 == | == 3D Structures of dimethylarginine dimethylaminohydrolase 1 == | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | [[3i2e]], [[6szq]] – hDDAH - human <br /> | + | [[3i2e]], [[6szq]], [[7usz]], [[7ut0]] – hDDAH - human <br /> |
[[2jai]] – hDDAH + citrulline <br /> | [[2jai]] – hDDAH + citrulline <br /> | ||
[[2jaj]], [[3p8e]], [[3i4a]] – hDDAH + ornithine derivative<br /> | [[2jaj]], [[3p8e]], [[3i4a]] – hDDAH + ornithine derivative<br /> | ||
| Line 64: | Line 64: | ||
[[3rhy]] – PaDDAH + chloro-hydroxymethylpyridine – ''Pseudomonas aeruginosa''<br /> | [[3rhy]] – PaDDAH + chloro-hydroxymethylpyridine – ''Pseudomonas aeruginosa''<br /> | ||
[[1h70]] – PaDDAH (mutant) + citrulline <br /> | [[1h70]] – PaDDAH (mutant) + citrulline <br /> | ||
| - | + | </StructureSection> | |
== References == | == References == | ||
{{reflist}} | {{reflist}} | ||
Revision as of 10:04, 24 January 2023
| |||||||||||
References
- ↑ 1.0 1.1 Palm F, Onozato ML, Luo Z, Wilcox CS. Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. American Journal of Physiology. 2007 Dec 1;293(6):3227-3245. PMID:17933965 doi:10.1152/ajpheart.00998.2007
- ↑ 2.0 2.1 2.2 Tran CTL, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atherosclerosis Supplements. 2003 Dec;4(4):33-40. PMID:14664901 doi:10.1016/S1567-5688(03)00032-1
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure. 2006 May;14(5):901-911. PMID:[1] doi:10.1016/j.str.2006.03.006
- ↑ Humm A, Fritsche E, Mann K, Göhl M, Huber R. Recombinant expression and isolation of human L-arginine:glycine amidinotransferase and identification of its active-site cysteine residue. Biochemical Journal. 1997 March 15;322(3):771-776. PMID:9148748 doi:10.1042/bj3220771
- ↑ 5.0 5.1 5.2 Rasheed M, Richter C, Chisty LT, Kirkpatrick J, Blackledge M, Webb MR, Driscoll PC. Ligand-dependent dynamics of the active site lid in bacterial Dimethyarginine Dimethylaminohydrolase. Biochemistry. 2014 Feb 18;53:1092-1104. PMCID:PMC3945819 doi:10.1021/bi4015924
- ↑ 6.0 6.1 6.2 Stone EM, Costello AL, Tierney DL, Fast W. Substrate-assisted cysteine deprotonation in the mechanism of Dimethylargininase (DDAH) from Pseudomonas aeruginosa. Biochemistry. 2006 May 2;45(17):5618-5630. PMID:16634643 doi:10.1021/bi052595m
- ↑ 7.0 7.1 Pace NJ, Weerpana E. Zinc-binding cysteines: diverse functions and structural motifs. Biomolecules. 2014 June;4(2):419-434. PMCID:4101490 doi:10.3390/biom4020419
- ↑ Janssen W, Pullamsetti SS, Cooke J, Weissmann N, Guenther A, Schermuly RT. The role of dimethylarginine dimethylaminohydrolase (DDAH) in pulmonary fibrosis. The Journal of Pathology. 2012 Dec 12;229(2):242-249. Epub 2013 Jan. PMID:23097221 doi:10.1002/path.4127
- ↑ Colasanti M, Suzuki H. The dual personality of NO. ScienceDirect. 2000 Jul 1;21(7):249-252. PMID:10979862 doi:10.1016/S0165-6147(00)01499-1
- ↑ Rassaf T, Feelisch M, Kelm M. Circulating NO pool: assessment of nitrite and nitroso species in blood and tissues. Free Rad. Biol. Med. 2004 Feb 15;36(4):413-422. PMID:14975444 doi:10.1016/j.freeradbiomed.2003.11.011
- ↑ Tsao PS, Cooke JP. Endothelial alterations in hypercholesterolemia: more than simply vasodilator dysfunction. Journal of Cardiovascular Pharmacology. 1998;32(3):48-53. PMID:9883748
- ↑ Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat. Rev. Drug Discov. 2002 Dec;1(12):939-950. PMID:12461516 doi:10.1038/nrd960
Student Contributors
- Natalie Van Ochten
- Kaitlyn Enderle
- Colton Junod
Proteopedia Page Contributors and Editors (what is this?)
Natalie Van Ochten, Michal Harel, Alexander Berchansky, Kaitlyn Enderle, Colton Junod, Joel L. Sussman