Indole-3-glycerol phosphate synthase
From Proteopedia
(Difference between revisions)
(New page: <StructureSection load='1a53' size='350' side='right' caption='Structure of IGPS complex with IGP (PDB entry 1a53)' scene=''> '''Indole-3-glycerol phosphate synthase''' (IGPS) ca...) |
|||
| (22 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='' size='350' side='right' caption='Structure of IGPS complex with IGP (PDB entry [[1a53]])' scene='57/572309/Cv/1'> | ||
| + | == Function == | ||
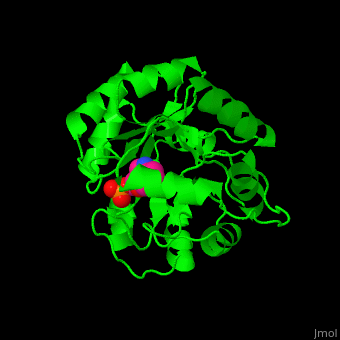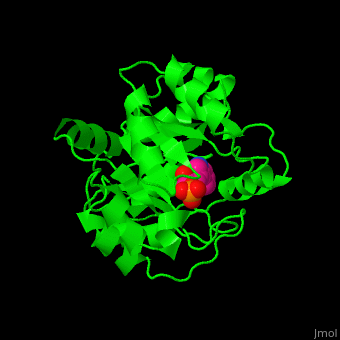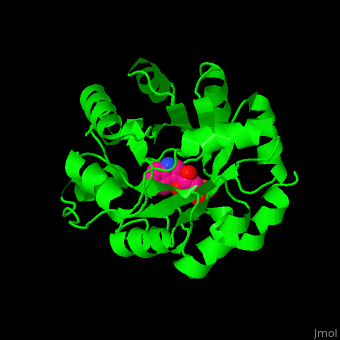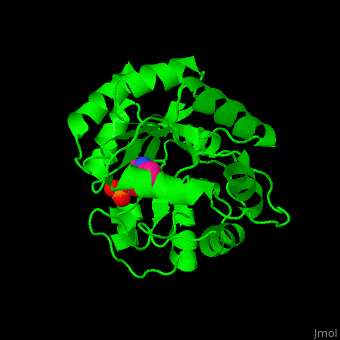
| + | '''Indole-3-glycerol phosphate synthase''' or '''imidazole glycerol phosphate synthase''' (IGPS) catalyzes the reversible transformation of 1-(2-carboxyphenylamino)-1-deoxy-D-ribulose 5-phosphate (CdRP) to 1-C-(indol-e-yl)-glycerol 3-phosphate (IGP), CO2 and H2O. IGPS is part of the tryptophan biosynthesis<ref>PMID:5332729</ref>. | ||
| - | + | == Structural highlights == | |
| - | + | The <scene name='57/572309/Cv/6'>active site of IGPS containing the reaction product IGP</scene><ref>PMID:12054868</ref>. Water molecules shown as red spheres. | |
| - | + | </StructureSection> | |
==3D structures of IGPS== | ==3D structures of IGPS== | ||
| - | + | [[IGPS 3D structures]] | |
| - | [[ | + | == References == |
| - | + | <references/> | |
| - | + | [[Category:Topic Page]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | == | + | |
| - | + | ||
| - | + | ||
| - | [[ | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
| |||||||||||
3D structures of IGPS
References
- ↑ Creighton TE, Yanofsky C. Indole-3-glycerol phosphate synthetase of Escherichia coli, an enzyme of the tryptophan operon. J Biol Chem. 1966 Oct 25;241(20):4616-24. PMID:5332729
- ↑ Hennig M, Darimont BD, Jansonius JN, Kirschner K. The catalytic mechanism of indole-3-glycerol phosphate synthase: crystal structures of complexes of the enzyme from Sulfolobus solfataricus with substrate analogue, substrate, and product. J Mol Biol. 2002 Jun 7;319(3):757-66. PMID:12054868 doi:http://dx.doi.org/10.1016/S0022-2836(02)00378-9