Ketosteroid Isomerase
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
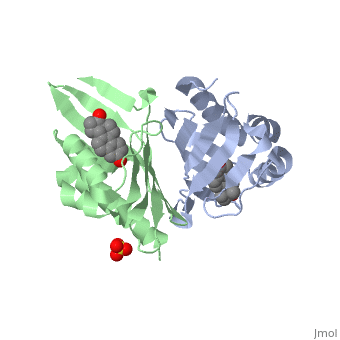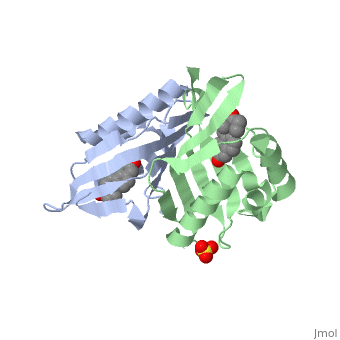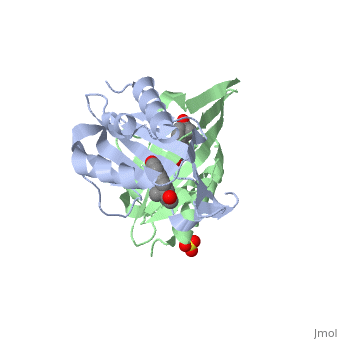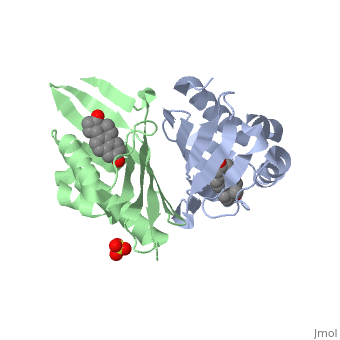
<StructureSection load='1qjg' size='350' side='right' scene='' caption='Ketosteroid isomerase complex with transition state analog equilenin and sulfate (PDB code [[1qjg]])'> | <StructureSection load='1qjg' size='350' side='right' scene='' caption='Ketosteroid isomerase complex with transition state analog equilenin and sulfate (PDB code [[1qjg]])'> | ||
==Introduction== | ==Introduction== | ||
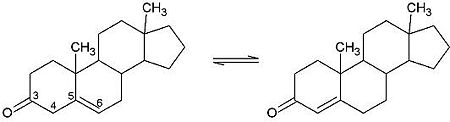
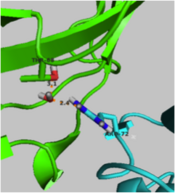
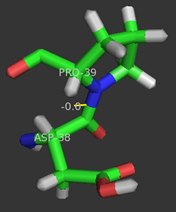
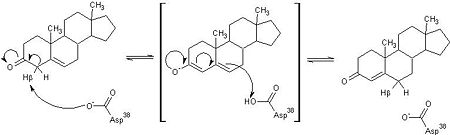
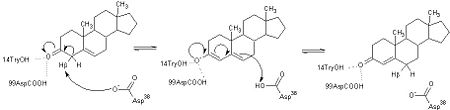
| - | <scene name='User:Laura_M._Haynes/Sandbox_1/Ksi/4'>Ketosteroid isomerase</scene> (KSI, EC#5.3.3.1) is an enzyme that catalyzes the isomerization of 3-oxo-Δ<sup>5</sup> ketosteroids to their hormonally active Δ<sup>4</sup>-conjugated isomers, as illustrated below.<ref name="Pollack">PMID:15381400</ref>, <ref name="Talalay">PMID:13276386 </ref> | + | <scene name='User:Laura_M._Haynes/Sandbox_1/Ksi/4'>Ketosteroid isomerase</scene> (KSI, EC#5.3.3.1) or '''steroid delta-isomerase''' is an enzyme that catalyzes the isomerization of 3-oxo-Δ<sup>5</sup> ketosteroids to their hormonally active Δ<sup>4</sup>-conjugated isomers, as illustrated below.<ref name="Pollack">PMID:15381400</ref>, <ref name="Talalay">PMID:13276386 </ref> |
[[Image:Reaction.jpg|left|450px|thumb]] | [[Image:Reaction.jpg|left|450px|thumb]] | ||
| Line 121: | Line 121: | ||
**[[8cho]] – CtKSI<BR /> | **[[8cho]] – CtKSI<BR /> | ||
**[[1ocv]], [[3nxj]], [[3mhe]], [[3mki]], [[3myt]], [[3nm2]], [[3t8u]], [[3unl]], [[4l7k]], [[4k1u]], [[4k1v]], [[5dre]] – CtKSI (mutant) <BR /> | **[[1ocv]], [[3nxj]], [[3mhe]], [[3mki]], [[3myt]], [[3nm2]], [[3t8u]], [[3unl]], [[4l7k]], [[4k1u]], [[4k1v]], [[5dre]] – CtKSI (mutant) <BR /> | ||
| - | **[[1opy]], [[3vsy]] – PpKSI – ''Pseudomonas putida''<BR /> | + | **[[1opy]], [[3vsy]], [[6u1z]], [[6ucw]] – PpKSI – ''Pseudomonas putida''<BR /> |
| - | **[[1c7h]], [[1dmm]], [[1dmn]], [[1dmq]], [[1e97]], [[1ea2]], [[1k41]], [[1vzz]], [[1w01]], [[1w02]], [[1w6y]], [[1w00]], [[3sed]], [[3ox9]], [[3oxa]], [[3t8n]], [[3rgr]], [[5d81]], [[5d82]], [[5d83]], [[6f4y]], [[6f50]], [[6f53]], [[6f54]] – PpKSI (mutant) <BR /> | + | **[[1c7h]], [[1dmm]], [[1dmn]], [[1dmq]], [[1e97]], [[1ea2]], [[1k41]], [[1vzz]], [[1w01]], [[1w02]], [[1w6y]], [[1w00]], [[3sed]], [[3ox9]], [[3oxa]], [[3t8n]], [[3rgr]], [[5d81]], [[5d82]], [[5d83]], [[6f4y]], [[6f50]], [[6f53]], [[6f54]], [[6uad]], [[6uae]], [[7rxf]], [[7rxk]] – PpKSI (mutant) <BR /> |
**[[5z3r]] – KSI – ''Mycobacterium neoaurum'' <BR /> | **[[5z3r]] – KSI – ''Mycobacterium neoaurum'' <BR /> | ||
| + | **[[6p3l]] – MhKSI – ''Mycobacterium hassiacum'' <BR /> | ||
| + | **[[7epn]] – MsKSI – ''Mycobacterium smegmatis'' <BR /> | ||
*Ketosteroid isomerase complexes | *Ketosteroid isomerase complexes | ||
| Line 132: | Line 134: | ||
**[[1qjg]] – PtKSI (mutant) + equilenin – ''Pseudomonas testosteroni''<BR /> | **[[1qjg]] – PtKSI (mutant) + equilenin – ''Pseudomonas testosteroni''<BR /> | ||
**[[1e3r]] – PpKSI (mutant) + androgen derivative <BR /> | **[[1e3r]] – PpKSI (mutant) + androgen derivative <BR /> | ||
| - | **[[1gs3]], [[1ogx]], [[1cqs]], [[1oho]], [[3fzw]], [[3ipt]], [[3ows]], [[3owu]], [[3owy]], [[5ai1]], [[5g2g]], [[5kp1]], [[5kp3]], [[5kp4]] – PpKSI (mutant) + equilenin <BR /> | ||
**[[1oh0]] – PpKSI + equilenin <BR /> | **[[1oh0]] – PpKSI + equilenin <BR /> | ||
| + | **[[1gs3]], [[1ogx]], [[1cqs]], [[1oho]], [[3fzw]], [[3ipt]], [[3ows]], [[3owu]], [[3owy]], [[5ai1]], [[5g2g]], [[5kp1]], [[5kp3]], [[5kp4]] – PpKSI (mutant) + equilenin <BR /> | ||
| + | **[[6tzd]], [[6ubq]], [[6ucy]] – PpKSI + androstenedione <BR /> | ||
| + | **[[6ufs]] – PpKSI + dihydronandrolone <BR /> | ||
| + | **[[6u4i]], [[6ucn]] – PpKSI + equilenin <BR /> | ||
**[[1e3v]], [[4cdl]] – PpKSI + inhibitor <BR /> | **[[1e3v]], [[4cdl]] – PpKSI + inhibitor <BR /> | ||
**[[2pzv]], [[2inx]], [[3cpo]], [[3vgn]], [[6c17]], [[6c1j]], [[6c1x]] – PpKSI (mutant) + phenol derivative<BR /> | **[[2pzv]], [[2inx]], [[3cpo]], [[3vgn]], [[6c17]], [[6c1j]], [[6c1x]] – PpKSI (mutant) + phenol derivative<BR /> | ||
| - | **[[1ohp]] – CtKSI (mutant) + | + | **[[7ry4]] – PpKSI (mutant) + transition state analog<BR /> |
| + | **[[6p44]] – MhKSI (mutant) + phenol derivative<BR /> | ||
| + | **[[1ohp]] – CtKSI (mutant) + estrogen derivative <BR /> | ||
**[[1ohs]] – CtKSI (mutant) + androgen derivative <BR /> | **[[1ohs]] – CtKSI (mutant) + androgen derivative <BR /> | ||
| + | **[[7epo]] – MsKSI + benzoxazole derivative <BR /> | ||
}} | }} | ||
==References== | ==References== | ||
Current revision
| |||||||||||
3D structures of ketosteroid isomerase
Updated on 13-June-2023
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Pollack RM. Enzymatic mechanisms for catalysis of enolization: ketosteroid isomerase. Bioorg Chem. 2004 Oct;32(5):341-53. PMID:15381400 doi:10.1016/j.bioorg.2004.06.005
- ↑ 2.0 2.1 TALALAY P, WANG VS. Enzymic isomerization of delta5-3-ketosteroids. Biochim Biophys Acta. 1955 Oct;18(2):300-1. PMID:13276386
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Ha NC, Choi G, Choi KY, Oh BH. Structure and enzymology of Delta5-3-ketosteroid isomerase. Curr Opin Struct Biol. 2001 Dec;11(6):674-8. PMID:11751047
- ↑ Ha NC, Choi G, Choi KY, Oh BH. Structure and enzymology of Delta5-3-ketosteroid isomerase. Curr Opin Struct Biol. 2001 Dec;11(6):674-8. PMID:11751047
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 Wu ZR, Ebrahimian S, Zawrotny ME, Thornburg LD, Perez-Alvarado GC, Brothers P, Pollack RM, Summers MF. Solution structure of 3-oxo-delta5-steroid isomerase. Science. 1997 Apr 18;276(5311):415-8. PMID:9103200
- ↑ 6.0 6.1 6.2 Murzin AG. How far divergent evolution goes in proteins. Curr Opin Struct Biol. 1998 Jun;8(3):380-7. PMID:9666335
- ↑ 7.0 7.1 Cleland WW, Frey PA, Gerlt JA. The low barrier hydrogen bond in enzymatic catalysis. J Biol Chem. 1998 Oct 2;273(40):25529-32. PMID:9748211
- ↑ 8.0 8.1 8.2 Kraut DA, Sigala PA, Pybus B, Liu CW, Ringe D, Petsko GA, Herschlag D. Testing electrostatic complementarity in enzyme catalysis: hydrogen bonding in the ketosteroid isomerase oxyanion hole. PLoS Biol. 2006 Apr;4(4):e99. Epub 2006 Mar 28. PMID:16602823 doi:10.1371/journal.pbio.0040099
- ↑ Cho HS, Choi G, Choi KY, Oh BH. Crystal structure and enzyme mechanism of Delta 5-3-ketosteroid isomerase from Pseudomonas testosteroni. Biochemistry. 1998 Jun 9;37(23):8325-30. PMID:9622484 doi:10.1021/bi9801614
- ↑ 10.0 10.1 Aurora R, Rose GD. Helix capping. Protein Sci. 1998 Jan;7(1):21-38. PMID:9514257 doi:10.1002/pro.5560070103
- ↑ 11.0 11.1 Kim DH, Jang DS, Nam GH, Choi KY. Folding mechanism of ketosteroid isomerase from Comamonas testosteroni. Biochemistry. 2001 Apr 24;40(16):5011-7. PMID:11305917
- ↑ Massiah MA, Abeygunawardana C, Gittis AG, Mildvan AS. Solution structure of Delta 5-3-ketosteroid isomerase complexed with the steroid 19-nortestosterone hemisuccinate. Biochemistry. 1998 Oct 20;37(42):14701-12. PMID:9778345 doi:10.1021/bi981447b
- ↑ Kim SW, Cha SS, Cho HS, Kim JS, Ha NC, Cho MJ, Joo S, Kim KK, Choi KY, Oh BH. High-resolution crystal structures of delta5-3-ketosteroid isomerase with and without a reaction intermediate analogue. Biochemistry. 1997 Nov 18;36(46):14030-6. PMID:9369474 doi:10.1021/bi971546+
- ↑ Sigala PA, Kraut DA, Caaveiro JM, Pybus B, Ruben EA, Ringe D, Petsko GA, Herschlag D. Testing geometrical discrimination within an enzyme active site: constrained hydrogen bonding in the ketosteroid isomerase oxyanion hole. J Am Chem Soc. 2008 Oct 15;130(41):13696-708. Epub 2008 Sep 23. PMID:18808119 doi:10.1021/ja803928m
- ↑ 15.0 15.1 Nam GH, Cha SS, Yun YS, Oh YH, Hong BH, Lee HS, Choi KY. The conserved cis-Pro39 residue plays a crucial role in the proper positioning of the catalytic base Asp38 in ketosteroid isomerase from Comamonas testosteroni. Biochem J. 2003 Oct 15;375(Pt 2):297-305. PMID:12852789 doi:10.1042/BJ20030263
- ↑ Cho HS, Ha NC, Choi G, Kim HJ, Lee D, Oh KS, Kim KS, Lee W, Choi KY, Oh BH. Crystal structure of delta(5)-3-ketosteroid isomerase from Pseudomonas testosteroni in complex with equilenin settles the correct hydrogen bonding scheme for transition state stabilization. J Biol Chem. 1999 Nov 12;274(46):32863-8. PMID:10551849
- ↑ 17.0 17.1 Zhao Q, Abeygunawardana C, Talalay P, Mildvan AS. NMR evidence for the participation of a low-barrier hydrogen bond in the mechanism of delta 5-3-ketosteroid isomerase. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8220-4. PMID:8710850
- ↑ Zhao Q, Abeygunawardana C, Talalay P, Mildvan AS. NMR evidence for the participation of a low-barrier hydrogen bond in the mechanism of delta 5-3-ketosteroid isomerase. Proc Natl Acad Sci U S A. 1996 Aug 6;93(16):8220-4. PMID:8710850
- ↑ 19.0 19.1 Kraut DA, Sigala PA, Fenn TD, Herschlag D. Dissecting the paradoxical effects of hydrogen bond mutations in the ketosteroid isomerase oxyanion hole. Proc Natl Acad Sci U S A. 2010 Feb 2;107(5):1960-5. Epub 2010 Jan 11. PMID:20080683
- ↑ Sigala PA, Caaveiro JM, Ringe D, Petsko GA, Herschlag D. Hydrogen bond coupling in the ketosteroid isomerase active site. Biochemistry. 2009 Jul 28;48(29):6932-9. PMID:19469568 doi:10.1021/bi900713j
- ↑ 21.0 21.1 Cherney MM, Garen CR, James MN. Crystal structure of Mycobacterium tuberculosis Rv0760c at 1.50 A resolution, a structural homolog of Delta(5)-3-ketosteroid isomerase. Biochim Biophys Acta. 2008 Nov;1784(11):1625-32. Epub 2008 Jun 6. PMID:18589008 doi:10.1016/j.bbapap.2008.05.012
Proteopedia Page Contributors and Editors (what is this?)
Laura M. Haynes, Michal Harel, Joel L. Sussman, Alexander Berchansky